当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The eukaryotic tRNA-guanine transglycosylase enzyme inserts queuine into tRNA via a sequential bi–bi mechanism
Chemical Communications ( IF 4.3 ) Pub Date : 2020/03/09 , DOI: 10.1039/c9cc09887a Mashael A. Alqasem 1, 2, 3, 4, 5 , Claire Fergus 1, 2, 3, 4, 5 , J. Mike Southern 2, 3, 4, 5, 6 , Stephen J. Connon 2, 3, 4, 5, 6 , Vincent P. Kelly 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2020/03/09 , DOI: 10.1039/c9cc09887a Mashael A. Alqasem 1, 2, 3, 4, 5 , Claire Fergus 1, 2, 3, 4, 5 , J. Mike Southern 2, 3, 4, 5, 6 , Stephen J. Connon 2, 3, 4, 5, 6 , Vincent P. Kelly 1, 2, 3, 4, 5
Affiliation
|
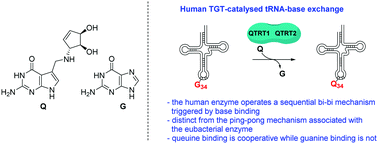
Eukaryotic tRNA-guanine transglycosylase (TGT) – an enzyme recently recognised to be of potential therapeutic importance – catalyses base-exchange of guanine for queuine at the wobble position of tRNAs associated with 4 amino acids via a distinct mechanism to that reported for its eubacterial homologue. The presence of queuine is unequivocally required as a trigger for reaction between the enzyme and tRNA and exhibits cooperativity not seen using guanine as a substrate.
中文翻译:

真核tRNA-鸟嘌呤转糖基酶通过连续的bi-bi机制将奎宁插入tRNA
真核tRNA-鸟嘌呤转糖基酶(TGT)–一种最近被认为具有潜在治疗意义的酶–通过与报道其真细菌同源性不同的机制,催化与4个氨基酸相关的tRNA摆动位置上鸟嘌呤的奎宁碱基交换。。无疑需要奎因的存在作为酶和tRNA之间反应的触发因素,并显示出使用鸟嘌呤作为底物所没有的协同作用。
更新日期:2020-04-03
中文翻译:

真核tRNA-鸟嘌呤转糖基酶通过连续的bi-bi机制将奎宁插入tRNA
真核tRNA-鸟嘌呤转糖基酶(TGT)–一种最近被认为具有潜在治疗意义的酶–通过与报道其真细菌同源性不同的机制,催化与4个氨基酸相关的tRNA摆动位置上鸟嘌呤的奎宁碱基交换。。无疑需要奎因的存在作为酶和tRNA之间反应的触发因素,并显示出使用鸟嘌呤作为底物所没有的协同作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号