当前位置:
X-MOL 学术
›
Nat. Rev. Nephrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The tubular hypothesis of nephron filtration and diabetic kidney disease.
Nature Reviews Nephrology ( IF 28.6 ) Pub Date : 2020-03-09 , DOI: 10.1038/s41581-020-0256-y Volker Vallon 1, 2, 3 , Scott C Thomson 1, 3
Nature Reviews Nephrology ( IF 28.6 ) Pub Date : 2020-03-09 , DOI: 10.1038/s41581-020-0256-y Volker Vallon 1, 2, 3 , Scott C Thomson 1, 3
Affiliation
|
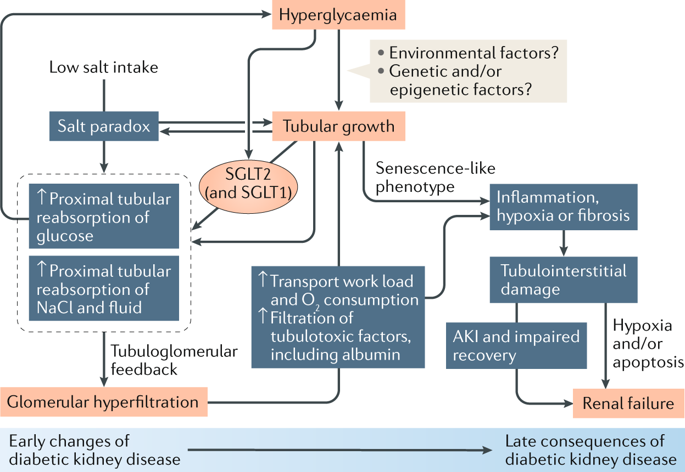
Kidney size and glomerular filtration rate (GFR) often increase with the onset of diabetes, and elevated GFR is a risk factor for the development of diabetic kidney disease. Hyperfiltration mainly occurs in response to signals passed from the tubule to the glomerulus: high levels of glucose in the glomerular filtrate drive increased reabsorption of glucose and sodium by the sodium-glucose cotransporters SGLT2 and SGLT1 in the proximal tubule. Passive reabsorption of chloride and water also increases. The overall capacity for proximal reabsorption is augmented by growth of the proximal tubule, which (alongside sodium-glucose cotransport) further limits urinary glucose loss. Hyperreabsorption of sodium and chloride induces tubuloglomerular feedback from the macula densa to increase GFR. In addition, sodium-glucose cotransport by SGLT1 on macula densa cells triggers the production of nitric oxide, which also contributes to glomerular hyperfiltration. Although hyperfiltration restores sodium and chloride excretion it imposes added physical stress on the filtration barrier and increases the oxygen demand to drive reabsorption. Tubular growth is associated with the development of a senescence-like molecular signature that sets the stage for inflammation and fibrosis. SGLT2 inhibitors attenuate the proximal reabsorption of sodium and glucose, normalize tubuloglomerular feedback signals and mitigate hyperfiltration. This tubule-centred model of diabetic kidney physiology predicts the salutary effect of SGLT2 inhibitors on hard renal outcomes, as shown in large-scale clinical trials.
中文翻译:

肾单位滤过和糖尿病性肾脏疾病的肾小管假说。
肾脏的大小和肾小球滤过率(GFR)通常随糖尿病的发作而增加,而GFR升高是糖尿病肾病发展的危险因素。超滤主要是响应于从肾小管传递至肾小球的信号而发生的:肾小球滤出液中的高水平葡萄糖促使近端肾小管中的钠-葡萄糖共转运蛋白SGLT2和SGLT1对葡萄糖和钠的重吸收增加。氯和水的被动重吸收也会增加。近端肾小管的生长增强了近端重吸收的总体能力,这进一步限制了尿葡萄糖的流失。钠和氯离子的过度吸收会引起黄斑部小管的肾小球反馈,从而增加GFR。此外,SGLT1在黄斑牙本质细胞上的钠-葡萄糖共转运触发了一氧化氮的产生,这也有助于肾小球超滤。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。这也有助于肾小球超滤。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。这也有助于肾小球超滤。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。
更新日期:2020-03-09
中文翻译:

肾单位滤过和糖尿病性肾脏疾病的肾小管假说。
肾脏的大小和肾小球滤过率(GFR)通常随糖尿病的发作而增加,而GFR升高是糖尿病肾病发展的危险因素。超滤主要是响应于从肾小管传递至肾小球的信号而发生的:肾小球滤出液中的高水平葡萄糖促使近端肾小管中的钠-葡萄糖共转运蛋白SGLT2和SGLT1对葡萄糖和钠的重吸收增加。氯和水的被动重吸收也会增加。近端肾小管的生长增强了近端重吸收的总体能力,这进一步限制了尿葡萄糖的流失。钠和氯离子的过度吸收会引起黄斑部小管的肾小球反馈,从而增加GFR。此外,SGLT1在黄斑牙本质细胞上的钠-葡萄糖共转运触发了一氧化氮的产生,这也有助于肾小球超滤。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。这也有助于肾小球超滤。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。这也有助于肾小球超滤。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。尽管超滤恢复了钠和氯的排泄,但它在过滤屏障上施加了额外的物理压力,并增加了对再吸收的需氧量。肾小管的生长与衰老样分子标记的发展有关,后者为炎症和纤维化奠定了基础。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。SGLT2抑制剂可减弱钠和葡萄糖的近端重吸收,使肾小管肾小管反馈信号正常化并减轻超滤现象。如大规模临床试验所示,这种以肾小管为中心的糖尿病肾脏生理模型可预测SGLT2抑制剂对坚硬肾脏结局的有益作用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号