Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-03-09 , DOI: 10.1038/s41419-020-2366-7 Yufan Zhou , Yun Huang , Kuan Hu , Zeyu Zhang , Jiajin Yang , Zhiming Wang
|
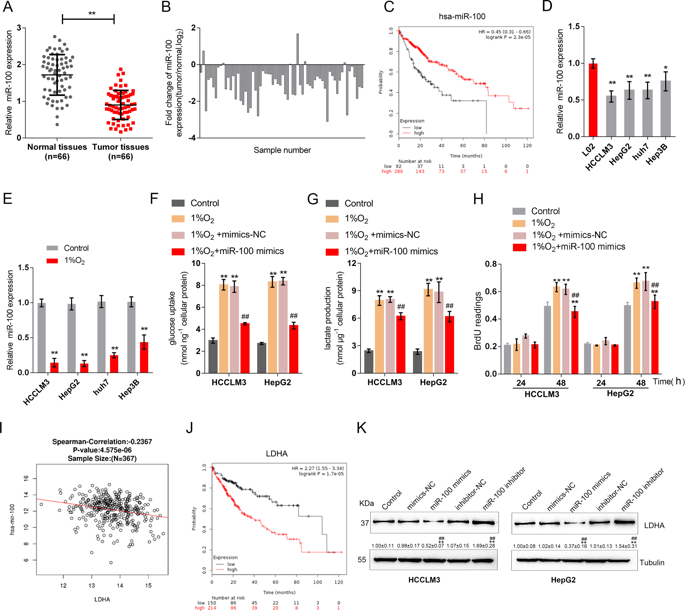
Hepatocellular carcinoma (HCC) remains the primary cause of cancer-related death. Metabolic change is the major characteristic of cancer. The present study attempted to investigate the regulatory mechanisms of HCC energy metabolism from the perspective of noncoding RNA regulation of HIF1A and LDHA. The expression of miR-100-5p expression was significantly suppressed in HCC tissue samples and HCC cell lines under 1% O2-induced hypoxia. miR-100-5p overexpression significantly suppressed hypoxia-induced increases in lactate concentration and glucose uptake. Exposure to 1% O2 induced HIF1A protein and reduced miR-100-5p expression, while HIF1A silencing dramatically rescued miR-100-5p expression upon 1% O2 exposure. In addition, 1% O2-induced increases in lactate concentration and glucose uptake were also suppressed by HIF1A silencing. Next, by analyzing available data in TCGA, we found that lncRNA RAET1K was correlated with HIF1A and miR-100-5p.LncRNA RAET1K could downregulate the expression of miR-100-5p by acting as a sponge, while HIF1A bound the lncRNA RAET1K promoter region to activate its transcription. LncRNA RAET1K silencing significantly suppressed HCC cell proliferation and invasion and also suppressed hypoxia-induced increases in lactate concentration and glucose uptake, while miR-100-5p inhibition reversed the effects of lncRNA RAET1K silencing on hypoxia-induced glycolysis in HCC cells. Finally, the expression of HIF1A, lncRNA RAET1K, and LDHA was upregulated in HCC tissue specimens; the expression of miR-100-5p was negatively related to HIF1A, lncRNA RAET1K, and LDHA; and HIF1A, lncRNA RAET1K, and LDHA were positively correlated with each other. In conclusion, the HIF1A/lncRNA RAET1K/miR-100-5p axis modulates hypoxia-induced glycolysis in HCC cells and might affect HCC progression.
中文翻译:

HIF1A通过miR-100-5p激活lncRNA RAET1K的转录以调节低氧诱导的肝癌细胞糖酵解
肝细胞癌(HCC)仍然是癌症相关死亡的主要原因。代谢变化是癌症的主要特征。本研究试图从HIF1A和LDHA的非编码RNA调控的角度研究HCC能量代谢的调控机制。在1%O 2诱导的缺氧条件下,HCC组织样品和HCC细胞系中miR-100-5p的表达被显着抑制。miR-100-5p的过表达显着抑制了缺氧引起的乳酸浓度和葡萄糖摄取的增加。暴露于1%氧气2诱导HIF1A蛋白和降低的miR-100-5p的表达,而在HIF1A 1%氧气的沉默显着地救出的miR-100-5p表达2曝光。另外1%O 2-HIF1A沉默也抑制了乳酸盐诱导的乳酸浓度和葡萄糖摄取增加。接下来,通过分析TCGA中的可用数据,我们发现lncRNA RAET1K与HIF1A和miR-100-5p相关.LncRNA RAET1K可以通过充当海绵来下调miR-100-5p的表达,而HIF1A结合lncRNA RAET1K启动子区域激活其转录。LncRNA RAET1K沉默显着抑制了HCC细胞的增殖和侵袭,并且还抑制了低氧诱导的乳酸浓度和葡萄糖摄取的增加,而miR-100-5p抑制作用则逆转了lncRNA RAET1K沉默对低氧诱导的HCC细胞糖酵解的影响。最后,在肝癌组织标本中HIF1A,lncRNA RAET1K和LDHA的表达上调。miR-100-5p的表达与HIF1A,lncRNA RAET1K呈负相关,和LDHA;HIF1A,lncRNA RAET1K和LDHA呈正相关。总之,HIF1A / lncRNA RAET1K / miR-100-5p轴可调节HCC细胞缺氧诱导的糖酵解,并可能影响HCC进程。









































 京公网安备 11010802027423号
京公网安备 11010802027423号