当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
p-Pyridinyl oxime carbamates: synthesis, DNA binding, DNA photocleaving activity and theoretical photodegradation studies
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-09 , DOI: 10.3762/bjoc.16.33 Panagiotis S Gritzapis 1 , Panayiotis C Varras 1 , Nikolaos-Panagiotis Andreou 1 , Katerina R Katsani 2 , Konstantinos Dafnopoulos 1, 3 , George Psomas 3 , Zisis V Peitsinis 4 , Alexandros E Koumbis 4 , Konstantina C Fylaktakidou 1, 4
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-09 , DOI: 10.3762/bjoc.16.33 Panagiotis S Gritzapis 1 , Panayiotis C Varras 1 , Nikolaos-Panagiotis Andreou 1 , Katerina R Katsani 2 , Konstantinos Dafnopoulos 1, 3 , George Psomas 3 , Zisis V Peitsinis 4 , Alexandros E Koumbis 4 , Konstantina C Fylaktakidou 1, 4
Affiliation
|
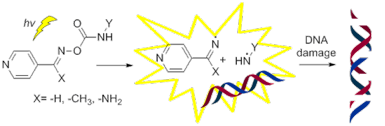
A number of p-pyridinyl oxime carbamate derivatives were prepared upon the reaction of the corresponding oximes with isocyanates. These novel compounds reacted photochemically in the presence of supercoiled plasmid DNA. Structure–activity relationship (SAR) studies revealed that the substituent on the imine group was not affecting the extend of the DNA damage, whereas the substituent of the carbamate group was critical, with the halogenated derivatives to be able to cause extensive single and double stranded DNA cleavages, acting as “synthetic nucleases”, independently of oxygen and pH. Calf thymus–DNA affinity studies showed a good-to-excellent affinity of selected both active and non-active derivatives. Preliminary theoretical studies were performed, in an effort to explain the reasons why some derivatives cause photocleavage and some others not, which were experimentally verified using triplet state activators and quenchers. These theoretical studies seem to allow the prediction of the activity of derivatives able to pass intersystem crossing to their triplet energy state and thus create radicals able to damage DNA. With this study, it is shown that oxime carbamate derivatives have the potential to act as novel effective photobase generating DNA-photocleavers, and are proposed as new leads for “on demand” biotechnological applications in drug discovery and medicine.
中文翻译:

对吡啶基肟氨基甲酸酯:合成、DNA 结合、DNA 光裂解活性和理论光降解研究
通过相应的肟与异氰酸酯的反应制备了许多对吡啶基肟氨基甲酸酯衍生物。这些新型化合物在超螺旋质粒 DNA 存在下发生光化学反应。构效关系(SAR)研究表明,亚胺基团上的取代基不会影响DNA损伤的程度,而氨基甲酸酯基团上的取代基则至关重要,卤代衍生物能够引起广泛的单链和双链损伤。 DNA 裂解,充当“合成核酸酶”,与氧气和 pH 无关。小牛胸腺-DNA 亲和力研究表明,选定的活性和非活性衍生物均具有良好至优异的亲和力。我们进行了初步的理论研究,试图解释为什么一些衍生物会引起光裂解,而另一些则不会,并使用三重态激活剂和猝灭剂进行了实验验证。这些理论研究似乎可以预测衍生物的活性,这些衍生物能够通过系间穿越达到三重态能量状态,从而产生能够损伤 DNA 的自由基。这项研究表明,肟氨基甲酸酯衍生物有潜力作为新型有效的光碱基生成 DNA 光切割剂,并被提议作为药物发现和医学中“按需”生物技术应用的新先导。
更新日期:2020-03-09
中文翻译:

对吡啶基肟氨基甲酸酯:合成、DNA 结合、DNA 光裂解活性和理论光降解研究
通过相应的肟与异氰酸酯的反应制备了许多对吡啶基肟氨基甲酸酯衍生物。这些新型化合物在超螺旋质粒 DNA 存在下发生光化学反应。构效关系(SAR)研究表明,亚胺基团上的取代基不会影响DNA损伤的程度,而氨基甲酸酯基团上的取代基则至关重要,卤代衍生物能够引起广泛的单链和双链损伤。 DNA 裂解,充当“合成核酸酶”,与氧气和 pH 无关。小牛胸腺-DNA 亲和力研究表明,选定的活性和非活性衍生物均具有良好至优异的亲和力。我们进行了初步的理论研究,试图解释为什么一些衍生物会引起光裂解,而另一些则不会,并使用三重态激活剂和猝灭剂进行了实验验证。这些理论研究似乎可以预测衍生物的活性,这些衍生物能够通过系间穿越达到三重态能量状态,从而产生能够损伤 DNA 的自由基。这项研究表明,肟氨基甲酸酯衍生物有潜力作为新型有效的光碱基生成 DNA 光切割剂,并被提议作为药物发现和医学中“按需”生物技术应用的新先导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号