Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.mcat.2020.110870 Caixia Cui , Hong Ming , Linjing Li , Mingjie Li , Jian Gao , Tao Han , Yunyun Wang
|
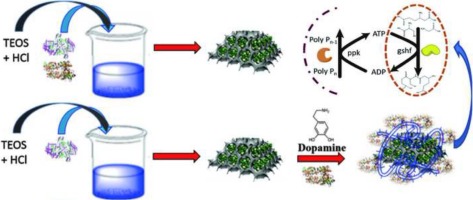
We report here adopting a glucose template for synthesizing mesoporous silica for enzyme in-situ immobilization. Bifunctional GSH synthetase (GSHF) and polyphosphate kinase (PPK) were selected as model system for synthesizing GSH with ATP regeneration. Results indicated that a pore size that was slightly smaller than that of enzyme was responsible for the high performance of immobilized enzyme inside mesoporous without protein leaking. Then, the cationic dopamine was used for PPK immobilization by self-polymerization. The co-immobilized enzyme activity was much higher than that of the free enzyme. The catalytic efficiency kcat/Km of co-immobilized enzyme was higher than that of the free enzyme (8.5 and 1.5 times higher for GSHF and PPK, respectively). After optimizing the reaction parameters, the co-immobilized enzymes exhibited excellent operational and storage stability, and could be re-used seven times and retained the original activity after 30 days. It is hoped that these findings will pave the way for applying enzyme co-immobilization to biocatalytic system.
中文翻译:

一个的制造原位在介孔二氧化硅共固定酶与ATP再生合成GSH
我们在这里报告采用葡萄糖模板来合成介孔二氧化硅,用于酶原位固定。选择双功能GSH合成酶(GSHF)和多磷酸激酶(PPK)作为模型系统,以ATP再生合成GSH。结果表明,孔径略小于酶的孔径是介孔内固定酶的高性能而不造成蛋白质泄漏的原因。然后,将阳离子多巴胺用于通过自聚合进行的PPK固定。固定化酶的活性远高于游离酶。催化效率kcat / Km固定化酶的游离蛋白含量高于游离酶(GSHF和PPK分别高8.5和1.5倍)。优化反应参数后,固定化酶表现出优异的操作和储存稳定性,可以重复使用7次,并在30天后保持原始活性。希望这些发现将为将酶共固定化应用于生物催化系统铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号