当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dehydrogenation versus hydrogenolysis in the reaction of light alkanes over Ni-based catalysts
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.jiec.2020.02.025 Guowei Wang , Shan Zhang , Xiaolin Zhu , Chunyi Li , Honghong Shan
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.jiec.2020.02.025 Guowei Wang , Shan Zhang , Xiaolin Zhu , Chunyi Li , Honghong Shan
|
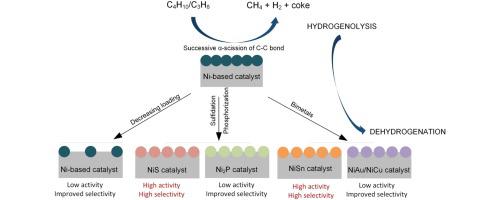
Abstract Given the high cost of Pt-based catalysts and the environmental issue of CrOx-based catalysts in dehydrogenation processes, Ni-based catalysts have been extensively explored as alternatives. In order to take the advantage of the high activation ability of Ni towards alkanes, herein, the reaction behaviors of light alkanes, primarily dehydrogenation versus hydrogenolysis, over Ni-based catalysts have been reviewed and probed. Ni-based catalysts exhibit an extremely high hydrogenolysis activity for light alkanes. Different from the single hydrogenolysis reaction on the surface of Pt, multiple hydrogenolysis of alkane molecules occurs over Ni sites. The successive α-scission of C C bond over Ni sites results in the generation of abundant methane and coke. To selectively activate C H bond of alkanes and prohibit C C bond rupture, one feasible approach is to introduce effective barriers to destroy the aggregated Ni ensembles active for hydrogenolysis. The promoting mechanism of different barriers (S, P, Cu, Sn, etc.) has been summarized and analyzed. The geometric effect of introduced barriers facilitates the dispersion of Ni particles, and electronic effect reduces the desorption energy of olefins and avoids undesired secondary reactions. Among all these catalysts, NiS and NiSn-based catalysts demonstrate the most outstanding dehydrogenation performance and show great potential for industrial applications.
中文翻译:

轻质烷烃在镍基催化剂上的脱氢与氢解反应
摘要 鉴于 Pt 基催化剂的高成本以及脱氢过程中 CrOx 基催化剂的环境问题,Ni 基催化剂已被广泛探索作为替代品。为了利用Ni对烷烃的高活化能力,本文对轻质烷烃在Ni基催化剂上的反应行为,主要是脱氢与氢解反应进行了综述和探讨。镍基催化剂对轻质烷烃表现出极高的氢解活性。与 Pt 表面的单次氢解反应不同,烷烃分子的多次氢解发生在 Ni 位点上。Ni 位点上 CC 键的连续 α 断裂导致大量甲烷和焦炭的产生。为了选择性地激活烷烃的 CH 键并阻止 CC 键断裂,一种可行的方法是引入有效的屏障来破坏对氢解具有活性的聚合 Ni 系综。对不同势垒(S、P、Cu、Sn等)的促进机理进行了总结和分析。引入势垒的几何效应促进了镍颗粒的分散,电子效应降低了烯烃的解吸能并避免了不需要的二次反应。在所有这些催化剂中,NiS和NiSn基催化剂表现出最出色的脱氢性能,并显示出巨大的工业应用潜力。引入势垒的几何效应促进了镍颗粒的分散,电子效应降低了烯烃的解吸能并避免了不需要的二次反应。在所有这些催化剂中,NiS和NiSn基催化剂表现出最出色的脱氢性能,并显示出巨大的工业应用潜力。引入势垒的几何效应促进了镍颗粒的分散,电子效应降低了烯烃的解吸能并避免了不需要的二次反应。在所有这些催化剂中,NiS和NiSn基催化剂表现出最出色的脱氢性能,并显示出巨大的工业应用潜力。
更新日期:2020-06-01
中文翻译:

轻质烷烃在镍基催化剂上的脱氢与氢解反应
摘要 鉴于 Pt 基催化剂的高成本以及脱氢过程中 CrOx 基催化剂的环境问题,Ni 基催化剂已被广泛探索作为替代品。为了利用Ni对烷烃的高活化能力,本文对轻质烷烃在Ni基催化剂上的反应行为,主要是脱氢与氢解反应进行了综述和探讨。镍基催化剂对轻质烷烃表现出极高的氢解活性。与 Pt 表面的单次氢解反应不同,烷烃分子的多次氢解发生在 Ni 位点上。Ni 位点上 CC 键的连续 α 断裂导致大量甲烷和焦炭的产生。为了选择性地激活烷烃的 CH 键并阻止 CC 键断裂,一种可行的方法是引入有效的屏障来破坏对氢解具有活性的聚合 Ni 系综。对不同势垒(S、P、Cu、Sn等)的促进机理进行了总结和分析。引入势垒的几何效应促进了镍颗粒的分散,电子效应降低了烯烃的解吸能并避免了不需要的二次反应。在所有这些催化剂中,NiS和NiSn基催化剂表现出最出色的脱氢性能,并显示出巨大的工业应用潜力。引入势垒的几何效应促进了镍颗粒的分散,电子效应降低了烯烃的解吸能并避免了不需要的二次反应。在所有这些催化剂中,NiS和NiSn基催化剂表现出最出色的脱氢性能,并显示出巨大的工业应用潜力。引入势垒的几何效应促进了镍颗粒的分散,电子效应降低了烯烃的解吸能并避免了不需要的二次反应。在所有这些催化剂中,NiS和NiSn基催化剂表现出最出色的脱氢性能,并显示出巨大的工业应用潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号