当前位置:
X-MOL 学术
›
J. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and asymmetric [4+2] cycloaddition reaction of 3,4,5-triphenyl-1-((1R,2S,5R)-menthyl)oxymethyl-1,2-diphosphole
Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.jorganchem.2020.121218 A.A. Zagidullin , Y.S. Ganushevich , V.A. Miluykov , P. Lönnecke , E. Hey-Hawkins
中文翻译:

3,4,5-三苯基-1-(((1R,2S,5R)-薄荷基)氧甲基-1,2-二膦酸酯)的合成和不对称[4 + 2]环加成反应
更新日期:2020-03-09
Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.jorganchem.2020.121218 A.A. Zagidullin , Y.S. Ganushevich , V.A. Miluykov , P. Lönnecke , E. Hey-Hawkins
|
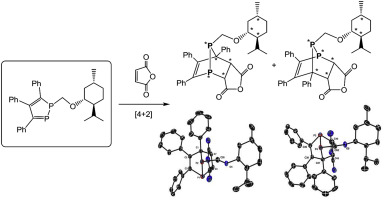
New chiral 3,4,5-triphenyl-1-((1R,2S,5R)-menthyl)oxymethyl-1,2-diphosphole (1) was prepared by reaction of sodium 1,2-diphospholide with chloromethyl (1R,2S,5R)-(−)-menthyl ether. The extent of asymmetric induction in the [4 + 2] cycloaddition reaction of chiral 1 with maleic anhydride was studied and the absolute configuration of the [4 + 2] cycloadducts determined by X-ray structure analysis.
中文翻译:

3,4,5-三苯基-1-(((1R,2S,5R)-薄荷基)氧甲基-1,2-二膦酸酯)的合成和不对称[4 + 2]环加成反应
新型手性3,4,5-三苯基-1-(((1 R,2 S,5 R)-薄荷基)氧甲基-1,2-二磷腈(1)是通过1,2-二磷化钠与氯甲基1 R,2 S,5 R)-(-)-薄荷基醚。研究了手性1与马来酸酐的[4 + 2]环加成反应中的不对称诱导程度,并通过X射线结构分析确定了[4 + 2]环加合物的绝对构型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号