当前位置:
X-MOL 学术
›
Neuropharmacology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disabling phosphorylation at the homer ligand of the metabotropic glutamate receptor 5 alleviates complete Freund's adjuvant-induced inflammatory pain.
Neuropharmacology ( IF 4.6 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.neuropharm.2020.108046 Limin Luo 1 , Min Huang 1 , Yu Zhang 1 , Wenying Wang 1 , Xiaqing Ma 1 , Haibo Shi 2 , Paul F Worley 3 , Dong Kwan Kim 4 , Sergei V Fedorovich 5 , Wei Jiang 1 , Tao Xu 6
Neuropharmacology ( IF 4.6 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.neuropharm.2020.108046 Limin Luo 1 , Min Huang 1 , Yu Zhang 1 , Wenying Wang 1 , Xiaqing Ma 1 , Haibo Shi 2 , Paul F Worley 3 , Dong Kwan Kim 4 , Sergei V Fedorovich 5 , Wei Jiang 1 , Tao Xu 6
Affiliation
|
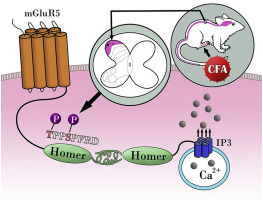
Metabotropic glutamate receptor 5 (mGluR5) has been reported to contribute to inflammatory pain. The intracellular C-terminal domain has a Homer-binding motif that can form an mGluR5/Homer complex. Phosphorylation of mGluR5 at the Homer binding domain enhances the mGluR5/Homer interaction and modulates intracellular signal transduction. However, the characteristics of this interaction have not been fully elucidated in inflammatory pain. We aimed to evaluate the effects of CFA-induced phosphorylation of mGluR5 at the Homer binding domain on the mGluR5/Homer interaction. Von-frey filaments and thermal latency were used to monitor the development of inflammatory pain. Spinal mGluR5 phosphorylation at Ser1126 and mGluR5/Homer crosslinking were detected. Mutant mGluR5 that could not be phosphorylated at Thr1123 or Ser1126 was evaluated in inflammatory pain. CFA-induced inflammatory pain resulted in obvious phosphorylation at Ser1126 of mGluR5. Moreover, increased phosphorylation at the Homer-binding motif enhanced crosslinking between mGluR5 and Homer. Mutations at Thr1123 and Ser1126 of mGluR5 blocked the development of CFA-induced inflammatory pain. Overall, our findings showed that disruption of the phosphorylation of mGluR5 Thr1123 and Ser1126 alleviated CFA-induced inflammatory pain.
中文翻译:

在代谢型谷氨酸受体5的荷马配体上禁用磷酸化可减轻弗氏完全佐剂诱导的炎症性疼痛。
据报道代谢型谷氨酸受体5(mGluR5)会引起炎症性疼痛。细胞内C端结构域具有可形成mGluR5 / Homer复合物的荷马结合基序。mGluR5在荷马结合域的磷酸化增强了mGluR5 / Homer的相互作用并调节细胞内信号转导。但是,这种相互作用的特征尚未在炎症性疼痛中得到充分阐明。我们旨在评估CFA诱导的荷马结合域上的mGluR5 /荷马相互作用的mGluR5磷酸化的影响。冯-弗雷丝和热潜伏期被用来监测炎症性疼痛的发展。检测到在Ser1126处的脊髓mGluR5磷酸化和mGluR5 / Homer交联。在炎性疼痛中评估了不能在Thr1123或Ser1126磷酸化的突变mGluR5。CFA引起的炎症性疼痛导致mGluR5的Ser1126出现明显的磷酸化。此外,在荷马结合基序处增加的磷酸化增强了mGluR5和荷马之间的交联。mGluR5的Thr1123和Ser1126处的突变阻止了CFA诱导的炎性疼痛的发展。总体而言,我们的发现表明,mGluR5 Thr1123和Ser1126磷酸化的破坏减轻了CFA引起的炎症性疼痛。
更新日期:2020-03-09
中文翻译:

在代谢型谷氨酸受体5的荷马配体上禁用磷酸化可减轻弗氏完全佐剂诱导的炎症性疼痛。
据报道代谢型谷氨酸受体5(mGluR5)会引起炎症性疼痛。细胞内C端结构域具有可形成mGluR5 / Homer复合物的荷马结合基序。mGluR5在荷马结合域的磷酸化增强了mGluR5 / Homer的相互作用并调节细胞内信号转导。但是,这种相互作用的特征尚未在炎症性疼痛中得到充分阐明。我们旨在评估CFA诱导的荷马结合域上的mGluR5 /荷马相互作用的mGluR5磷酸化的影响。冯-弗雷丝和热潜伏期被用来监测炎症性疼痛的发展。检测到在Ser1126处的脊髓mGluR5磷酸化和mGluR5 / Homer交联。在炎性疼痛中评估了不能在Thr1123或Ser1126磷酸化的突变mGluR5。CFA引起的炎症性疼痛导致mGluR5的Ser1126出现明显的磷酸化。此外,在荷马结合基序处增加的磷酸化增强了mGluR5和荷马之间的交联。mGluR5的Thr1123和Ser1126处的突变阻止了CFA诱导的炎性疼痛的发展。总体而言,我们的发现表明,mGluR5 Thr1123和Ser1126磷酸化的破坏减轻了CFA引起的炎症性疼痛。











































 京公网安备 11010802027423号
京公网安备 11010802027423号