当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coating biopolymer nanofibers with carbon nanotubes accelerates tissue healing and bone regeneration through orchestrated cell- and tissue-regulatory responses.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-03-09 , DOI: 10.1016/j.actbio.2020.03.012 Kapil D Patel 1 , Tae-Hyun Kim 2 , Nandin Mandakhbayar 2 , Rajendra K Singh 2 , Jun-Hyeog Jang 3 , Jung-Hwan Lee 4 , Hae-Won Kim 4
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-03-09 , DOI: 10.1016/j.actbio.2020.03.012 Kapil D Patel 1 , Tae-Hyun Kim 2 , Nandin Mandakhbayar 2 , Rajendra K Singh 2 , Jun-Hyeog Jang 3 , Jung-Hwan Lee 4 , Hae-Won Kim 4
Affiliation
|
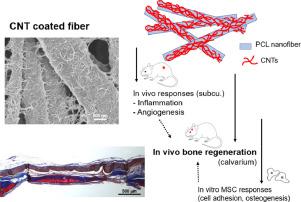
Tailoring the surface of biomaterial scaffolds has been a key strategy to modulate the cellular interactions that are helpful for tissue healing process. In particular, nanotopological surfaces have been demonstrated to regulate diverse behaviors of stem cells, such as initial adhesion, spreading and lineage specification. Here, we tailor the surface of biopolymer nanofibers with carbon nanotubes (CNTs) to create a unique bi-modal nanoscale topography (500 nm nanofiber with 25 nm nanotubes) and report the performance in modulating diverse in vivo responses including inflammation, angiogenesis, and bone regeneration. When administered to a rat subcutaneous site, the CNT-coated nanofiber exhibited significantly reduced inflammatory signs (down-regulated pro-inflammatory cytokines and macrophages gathering). Moreover, the CNT-coated nanofibers showed substantially promoted angiogenic responses, with enhanced neoblood vessel formation and angiogenic marker expression. Such stimulated tissue healing events by the CNT interfacing were evidenced in a calvarium bone defect model. The in vivo bone regeneration of the CNT- coated nanofibers was significantly accelerated, with higher bone mineral density and up-regulated osteogenic signs (OPN, OCN, BMP2) of in vivo bone forming cells. The in vitro studies using MSCs could demonstrate accelerated adhesion and osteogenic differentiation and mineralization, supporting the osteo-promoting mechanism behind the in vivo bone forming event. These findings highlight that the CNTs interfacing of biopolymer nanofibers is highly effective in reducing inflammation, promoting angiogenesis, and driving adhesion and osteogenesis of MSCs, which eventually orchestrate to accelerate tissue healing and bone regeneration process. STATEMENT OF SIGNIFICANCE: Here we demonstrate that the interfacing of biopolymer nanofibers with carbon nanotubes (CNTs) could modulate multiple interactions of cells and tissues that are ultimately helpful for the tissue healing and bone regeneration process. The CNT-coated scaffolds significantly reduced the pro-inflammatory signals while stimulating the angiogenic marker expressions. Furthermore, the CNT-coated scaffolds increased the bone matrix production of bone forming cells in vivo as well as accelerated the adhesion and osteogenic differentiation of MSCs in vitro. These collective findings highlight that the CNTs coated on the biopolymer nanofibers allow the creation of a promising platform for nanoscale engineering of biomaterial surface that can favor tissue healing and bone regeneration process, through a series of orchestrated events in anti-inflammation, pro-angiogenesis, and stem cell stimulation.
中文翻译:

带有碳纳米管的生物聚合物纳米纤维涂层可通过协调的细胞和组织调节反应来加速组织愈合和骨骼再生。
定制生物材料支架的表面一直是调节有助于组织愈合过程的细胞相互作用的关键策略。特别地,已经证明纳米拓扑表面可调节干细胞的多种行为,例如初始粘附,扩散和谱系规格。在这里,我们使用碳纳米管(CNT)定制生物聚合物纳米纤维的表面,以创建独特的双峰纳米级形貌(500 nm纳米纤维和25 nm纳米管),并报告其在调节包括炎症,血管生成和骨骼在内的多种体内反应中的性能。再生。当对大鼠皮下部位给药时,涂有CNT的纳米纤维表现出明显减少的炎症迹象(下调的促炎细胞因子和巨噬细胞聚集)。此外,碳纳米管包覆的纳米纤维显示出显着促进的血管生成反应,并增强了新血管的形成和血管生成标记的表达。在颅骨缺损模型中证明了这种通过CNT界面刺激的组织愈合事件。CNT涂覆的纳米纤维的体内骨骼再生得到显着加速,体内骨骼形成细胞的骨矿物质密度更高且成骨信号(OPN,OCN,BMP2)上调。使用MSC进行的体外研究可以证明其加速的粘附以及成骨细胞的分化和矿化,支持体内骨骼形成事件背后的骨促进机制。这些发现表明,生物聚合物纳米纤维的CNT界面在减少炎症,促进血管生成,并推动MSC的粘附和成骨,最终编排以加速组织愈合和骨骼再生过程。意义声明:在这里,我们证明生物聚合物纳米纤维与碳纳米管(CNT)的接口可以调节细胞和组织的多种相互作用,最终有助于组织愈合和骨骼再生过程。碳纳米管涂层的支架在刺激血管生成标记物表达的同时显着降低了促炎信号。此外,CNT涂层的支架在体内增加了骨形成细胞的骨基质产量,并在体外加速了MSC的粘附和成骨分化。
更新日期:2020-03-09
中文翻译:

带有碳纳米管的生物聚合物纳米纤维涂层可通过协调的细胞和组织调节反应来加速组织愈合和骨骼再生。
定制生物材料支架的表面一直是调节有助于组织愈合过程的细胞相互作用的关键策略。特别地,已经证明纳米拓扑表面可调节干细胞的多种行为,例如初始粘附,扩散和谱系规格。在这里,我们使用碳纳米管(CNT)定制生物聚合物纳米纤维的表面,以创建独特的双峰纳米级形貌(500 nm纳米纤维和25 nm纳米管),并报告其在调节包括炎症,血管生成和骨骼在内的多种体内反应中的性能。再生。当对大鼠皮下部位给药时,涂有CNT的纳米纤维表现出明显减少的炎症迹象(下调的促炎细胞因子和巨噬细胞聚集)。此外,碳纳米管包覆的纳米纤维显示出显着促进的血管生成反应,并增强了新血管的形成和血管生成标记的表达。在颅骨缺损模型中证明了这种通过CNT界面刺激的组织愈合事件。CNT涂覆的纳米纤维的体内骨骼再生得到显着加速,体内骨骼形成细胞的骨矿物质密度更高且成骨信号(OPN,OCN,BMP2)上调。使用MSC进行的体外研究可以证明其加速的粘附以及成骨细胞的分化和矿化,支持体内骨骼形成事件背后的骨促进机制。这些发现表明,生物聚合物纳米纤维的CNT界面在减少炎症,促进血管生成,并推动MSC的粘附和成骨,最终编排以加速组织愈合和骨骼再生过程。意义声明:在这里,我们证明生物聚合物纳米纤维与碳纳米管(CNT)的接口可以调节细胞和组织的多种相互作用,最终有助于组织愈合和骨骼再生过程。碳纳米管涂层的支架在刺激血管生成标记物表达的同时显着降低了促炎信号。此外,CNT涂层的支架在体内增加了骨形成细胞的骨基质产量,并在体外加速了MSC的粘附和成骨分化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号