Redox Biology ( IF 10.7 ) Pub Date : 2020-03-09 , DOI: 10.1016/j.redox.2020.101501 Yang Yang 1 , Jiayu Wang 2 , Shuyuan Guo 1 , Shirin Pourteymour 3 , Qiulian Xu 1 , Jie Gong 2 , Zhen Huang 1 , Zhaoqian Shen 1 , Kamal Diabakte 1 , Zhengyu Cao 1 , Guodong Wu 1 , Sukhareva Natalia 1 , Zhen Tian 2 , Hong Jin 3 , Ye Tian 4
|
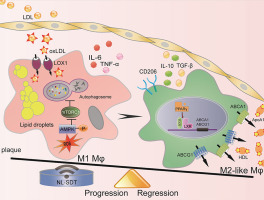
Emerging evidence indicates that macrophage functional polarization is critically involved in the development of atherosclerosis (AS). Here, we examined the role of 5-aminolaevulinic acid (ALA)-mediated non-lethal sonodynamic therapy (NL-SDT) in macrophage-subset polarization and atherosclerotic lesion stability and explored the potential underlying mechanisms. Using Western diet-fed apolipoprotein E (apoE)−/− and green fluorescent protein (GFP)-positive bone marrow (BM) chimeric mouse models, we demonstrated that NL-SDT promoted phenotypic switching of both BM-derived and resident macrophages from M1 to M2 and significantly inhibited AS progression. Further mechanistic studies indicated that NL-SDT enhanced macrophage differentiation toward the M2 phenotype by activating the reactive oxygen species (ROS)–5′ AMP-activated protein kinase (AMPK)–mammalian target of rapamycin complex 1 (mTORC1)–autophagy signaling pathway in murine BM-derived M1 macrophages (BMDM1s). Moreover, NL-SDT drastically reduced lipid droplets, mainly by promoting apoAI-mediated cholesterol efflux in vitro. Specifically, administration of pharmacological inhibitors to the animal model showed a reciprocal effect on NL-SDT-induced macrophage polarization. These findings indicate that NL-SDT engages a virtuous cycle that enhances M1-to-M2 polarization, cholesterol efflux, and anti-inflammatory reactions in advanced plaque in vivo and in BMDM1s in vitro by activating the ROS–AMPK–mTORC1–autophagy pathway. This discovery might help elucidate the mechanism underlying NL-SDT as a potential treatment to prevent atherothrombotic events.
中文翻译:

非致命声波动力疗法通过激活ROS-AMPK-mTORC1-自噬途径,促进了晚期动脉粥样硬化斑块中M1-M2的转变。
新兴证据表明,巨噬细胞功能极化与动脉粥样硬化(AS)的发展至关重要。在这里,我们检查了5-氨基松香酸(ALA)介导的非致死声波动力疗法(NL-SDT)在巨噬细胞亚群极化和动脉粥样硬化病变稳定性中的作用,并探讨了潜在的潜在机制。使用西方饮食喂养的载脂蛋白E(apoE)-/-和绿色荧光蛋白(GFP)阳性骨髓(BM)嵌合小鼠模型,我们证明了NL-SDT促进了BM来源的和驻留的巨噬细胞从M1到M2的表型转换,并显着抑制了AS的进展。进一步的机理研究表明,NL-SDT通过激活活性氧(ROS)-5'AMP激活的蛋白激酶(AMPK)-雷帕霉素复合物1(mTORC1)-自噬信号转导途径,增强了巨噬细胞向M2表型的分化。鼠BM衍生的M1巨噬细胞(BMDM1s)。另外,NL-SDT急剧降低脂滴,主要通过促进的apoAⅠ介导的胆固醇流出体外。具体而言,将药理抑制剂施用于动物模型显示出对NL-SDT诱导的巨噬细胞极化的相互影响。这些结果表明,NL-SDT接合良性循环增强M1至M2极化,胆固醇流出,并且在高级斑块抗炎反应在体内和在BMDM1s体外通过激活ROS-AMPK-的mTORC1的自噬途径。这一发现可能有助于阐明NL-SDT的潜在机制,作为预防动脉粥样硬化血栓形成事件的潜在治疗方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号