Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.jechem.2020.03.009 Yuan Cao , Jee-Jay J. Chen , Mark A. Barteau
|
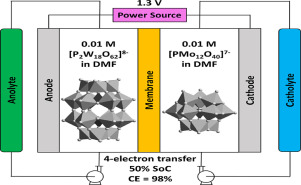
Polyoxometalates have been explored as multi-electron active species in both aqueous and non-aqueous redox flow batteries. Although non-aqueous systems in principle offer a wider voltage window for redox flow battery operation, realization of this potential requires a judicious choice of solvent as well as polyoxometalate properties. We demonstrate here the superior performance of N,N-dimethylformamide (DMF) compared to acetonitrile as a solvent for redox flow batteries based on Li3PMo12O40. This compound displays two 1-electron transfers in acetonitrile but can access an extra quasi-reversible 2-electron redox process in DMF. A cell containing 10 mM solution of Li3PMo12O40 in DMF produced a cell voltage of 0.7 V with 2-electron transfers (State of Charge = 60%) and showed a good cyclability. As a means to boost energy density, operation of the redox flow battery at a higher concentration of 0.1 M Li3PMo12O40 produced cells with cell voltage of 0.6 V in acetonitrile and a cell voltage of 1.0 V in DMF; both showed excellent coulombic efficiencies of more than 90% over the course of 30 cycles. Energy density was also increased by employing an asymmetric cell with different polyoxometalates on each side to extend cell voltage. Li6P2W18O62 exhibited 3 quasi-reversible 2-electron transfers in the potential range between −2.05 V and −0.5 V vs. Ag/Ag+. 10 mM Li6P2W18O62/Li3PMo12O40 in DMF produced a cell with cell voltage of 1.3 V involving 4-electron transfers (State of Charge = 50%) with coulombic efficiency of nearly 100% and energy efficiency of nearly 70% throughout the test with more than 20 cycles. These promising results demonstrate proof-of-concept approaches to improving the performance of polyoxometalates in non-aqueous redox flow batteries.
中文翻译:

提高非水氧化还原液流电池中多金属氧酸盐性能的系统方法
在水和非水氧化还原液流电池中都已将多金属氧酸盐开发为多电子活性物质。尽管原则上非水系统为氧化还原液流电池操作提供了更宽的电压窗口,但要实现这一潜力,需要明智地选择溶剂以及多金属氧酸盐的性能。我们在这里证明了N,N-二甲基甲酰胺(DMF)优于乙腈作为基于Li 3 PMo 12 O 40的氧化还原液流电池的溶剂。该化合物在乙腈中显示两个1电子转移,但可以在DMF中进行额外的准可逆2电子氧化还原过程。包含10 mM Li 3 PMo 12 O 40溶液的电池在DMF中产生2个电子转移(充电状态= 60%)时,电池电压为0.7 V,并显示出良好的循环性。作为提高能量密度的一种方法,在较高浓度的0.1 M Li 3 PMo 12 O 40下运行氧化还原液流电池时,电池中乙腈的电池电压为0.6 V,DMF中的电池电压为1.0V。在30个循环的过程中,两者均显示出超过90%的出色的库仑效率。通过使用两侧各不相同的多金属氧酸盐的不对称电池来扩展电池电压,还可以提高能量密度。Li 6 P 2 W 18 O 62与Ag / Ag +相比,在-2.05 V和-0.5 V之间的电位范围内,具有3个准可逆的2电子转移。DMF中的10 mM Li 6 P 2 W 18 O 62 / Li 3 PMo 12 O 40产生了一个电池,电压为1.3 V,涉及4电子转移(荷电状态= 50%),库仑效率接近100%,能量在超过20个周期的整个测试过程中,效率接近70%。这些有希望的结果证明了概念证明方法可改善非水氧化还原液流电池中多金属氧酸盐的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号