当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of human lactoferricin derived antitumor peptides-activity and specificity against malignant melanoma in 2D and 3D model studies.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.bbamem.2020.183264 Sarah Grissenberger 1 , Sabrina Riedl 2 , Beate Rinner 3 , Regina Leber 4 , Dagmar Zweytick 5
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-07 , DOI: 10.1016/j.bbamem.2020.183264 Sarah Grissenberger 1 , Sabrina Riedl 2 , Beate Rinner 3 , Regina Leber 4 , Dagmar Zweytick 5
Affiliation
|
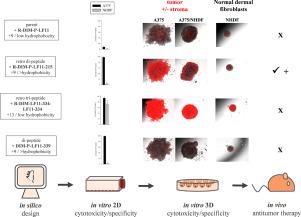
The aim of this study was to develop effective and specific anti-cancer drugs based on membrane active peptides. In previous studies we showed that human lactoferricin (hLFcin) derived peptides facilitate specific killing of cancer cells. These antitumor peptides were found by conventional melanoma two-dimensional (2D) cell cultures to induce apoptosis of cancer cells and to specifically target lipid phosphatidylserine located on the outside of cancer cell membranes. In order to have a more relevant in vitro model able to mimic the natural microenvironments of tumor tissues we established three-dimensional (3D) multicellular tumor spheroids (MCTS). We used a set of (retro) di-peptides derived from LF11, an 11 amino acid long fragment of hLFcin, which differed in peptide length, positive net charge and hydrophobicity and determined antitumor activity and non-specific toxicity on non-neoplastic cells using 2D and 3D model systems. 2D studies unveiled a correlation between length, positive net charge and hydrophobicity of peptides and their specific antitumor activity. (Retro) di-peptides as R-DIM-P-LF11-215 and DIM-LF11-322 with a net charge of +9 and moderate hydrophobicity exhibited the highest specific antitumor activity. Further evaluation of the peptides anticancer activity by 3D in vitro studies confirmed their higher activity and cancer specificity compared to their parent R-DIM-P-LF11, with the exception of DIM-LF11-339. This highly hydrophobic peptide caused cell death mainly at the border of tumor spheroids indicating that too high hydrophobicity may prevent peptides from reaching the center of the spheroids.
中文翻译:

在2D和3D模型研究中设计人乳铁蛋白衍生抗肿瘤肽的活性和针对恶性黑色素瘤的特异性。
这项研究的目的是开发基于膜活性肽的有效且特异性的抗癌药物。在以前的研究中,我们表明人乳铁蛋白(hLFcin)衍生的肽可促进癌细胞的特异性杀伤。这些抗肿瘤肽是通过常规的黑色素瘤二维(2D)细胞培养物发现的,可诱导癌细胞凋亡并特异性靶向位于癌细胞膜外侧的脂质磷脂酰丝氨酸。为了拥有能够模拟肿瘤组织自然微环境的更相关的体外模型,我们建立了三维(3D)多细胞肿瘤球体(MCTS)。我们使用了一组源自LF11的(复古)二肽,LF11是hLFcin的11个氨基酸长的片段,其肽段长度有所不同,使用2D和3D模型系统,对非肿瘤细胞具有正的净电荷和疏水性,并确定了抗肿瘤活性和非特异性毒性。2D研究揭示了肽的长度,正净电荷和疏水性与其特异性抗肿瘤活性之间的相关性。(复古)二肽R-DIM-P-LF11-215和DIM-LF11-322具有+9的净电荷和适度的疏水性,表现出最高的抗肿瘤活性。通过3D体外研究进一步评估了该肽的抗癌活性,证实了其母体R-DIM-P-LF11除DIM-LF11-339外具有更高的活性和癌症特异性。这种高度疏水性的肽主要在肿瘤球体的边界处引起细胞死亡,这表明疏水性太高可能会阻止肽到达球体的中心。
更新日期:2020-03-07
中文翻译:

在2D和3D模型研究中设计人乳铁蛋白衍生抗肿瘤肽的活性和针对恶性黑色素瘤的特异性。
这项研究的目的是开发基于膜活性肽的有效且特异性的抗癌药物。在以前的研究中,我们表明人乳铁蛋白(hLFcin)衍生的肽可促进癌细胞的特异性杀伤。这些抗肿瘤肽是通过常规的黑色素瘤二维(2D)细胞培养物发现的,可诱导癌细胞凋亡并特异性靶向位于癌细胞膜外侧的脂质磷脂酰丝氨酸。为了拥有能够模拟肿瘤组织自然微环境的更相关的体外模型,我们建立了三维(3D)多细胞肿瘤球体(MCTS)。我们使用了一组源自LF11的(复古)二肽,LF11是hLFcin的11个氨基酸长的片段,其肽段长度有所不同,使用2D和3D模型系统,对非肿瘤细胞具有正的净电荷和疏水性,并确定了抗肿瘤活性和非特异性毒性。2D研究揭示了肽的长度,正净电荷和疏水性与其特异性抗肿瘤活性之间的相关性。(复古)二肽R-DIM-P-LF11-215和DIM-LF11-322具有+9的净电荷和适度的疏水性,表现出最高的抗肿瘤活性。通过3D体外研究进一步评估了该肽的抗癌活性,证实了其母体R-DIM-P-LF11除DIM-LF11-339外具有更高的活性和癌症特异性。这种高度疏水性的肽主要在肿瘤球体的边界处引起细胞死亡,这表明疏水性太高可能会阻止肽到达球体的中心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号