当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-temperature decomposition of N2O from the HNO3 production: Process feasibility using a structured catalyst
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.ces.2020.115624 Milan Bernauer , Bohumil Bernauer , Galina Sádovská , Zdeněk Sobalík
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.ces.2020.115624 Milan Bernauer , Bohumil Bernauer , Galina Sádovská , Zdeněk Sobalík
|
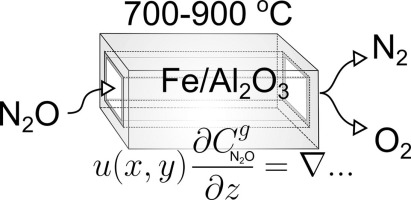
Abstract The high-temperature decomposition of N2O in a structured catalyst was studied using a full three-dimensional (3D) model in the temperature range 700–900 °C and high pressure (0.4–0.8 MPa), under realistic gas phase composition, i.e. with 1000 ppm of N2O, high concentrations of NO (9.5–10.5 mol%), O2 (4.5–5.0 mol%), and H2O (15.5–16.8 mol%). The kinetic data gathered from the previous study of the FeOx/Al2O3 catalyst displaying only medium activity were used as the input data. A single-channel 3D model without additional simplifications of fluid flow patterns and mass transfer in fluid and solid catalyst phases was employed to investigate the potential performance of a monolith reactor for N2O decomposition at high temperature regime. The model clearly predicted a relevant performance of the optimized honeycomb system based on such FeOx/Al2O3 catalyst.
中文翻译:

HNO3 生产中 N2O 的高温分解:使用结构化催化剂的工艺可行性
摘要 使用全三维 (3D) 模型在 700–900 °C 和高压 (0.4–0.8 MPa) 条件下,在真实的气相组成下研究了 N2O 在结构化催化剂中的高温分解,即含有 1000 ppm N2O、高浓度 NO(9.5-10.5 mol%)、O2(4.5-5.0 mol%)和 H2O(15.5-16.8 mol%)。从先前对仅显示中等活性的 FeOx/Al2O3 催化剂的研究中收集的动力学数据用作输入数据。没有额外简化流体流动模式和流体和固体催化剂相中的传质的单通道 3D 模型被用来研究整体反应器在高温状态下 N2O 分解的潜在性能。
更新日期:2020-07-01
中文翻译:

HNO3 生产中 N2O 的高温分解:使用结构化催化剂的工艺可行性
摘要 使用全三维 (3D) 模型在 700–900 °C 和高压 (0.4–0.8 MPa) 条件下,在真实的气相组成下研究了 N2O 在结构化催化剂中的高温分解,即含有 1000 ppm N2O、高浓度 NO(9.5-10.5 mol%)、O2(4.5-5.0 mol%)和 H2O(15.5-16.8 mol%)。从先前对仅显示中等活性的 FeOx/Al2O3 催化剂的研究中收集的动力学数据用作输入数据。没有额外简化流体流动模式和流体和固体催化剂相中的传质的单通道 3D 模型被用来研究整体反应器在高温状态下 N2O 分解的潜在性能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号