Chem ( IF 23.5 ) Pub Date : 2020-03-09 , DOI: 10.1016/j.chempr.2020.02.006 Andrew W Heard 1 , Stephen M Goldup 1
|
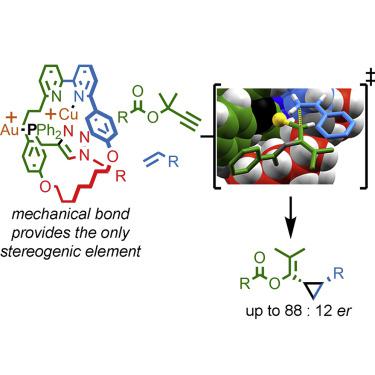
Rotaxanes are interlocked molecules in which a molecular ring is trapped on a dumbbell-shaped axle because of its inability to escape over the bulky end groups, resulting in a so-called mechanical bond. Interlocked molecules have mainly been studied as components of molecular machines, but the crowded, flexible environment created by threading one molecule through another has also been explored in catalysis and sensing. However, so far, the applications of one of the most intriguing properties of interlocked molecules, their ability to display stereogenic units that do not rely on the stereochemistry of their covalent subunits, termed “mechanical chirality,” have yet to be properly explored, and prototypical demonstration of the applications of mechanically chiral rotaxanes remain scarce. Here, we describe a mechanically planar chiral rotaxane-based Au complex that mediates a cyclopropanation reaction with stereoselectivities that are comparable with the best conventional covalent catalyst reported for this reaction.
中文翻译:

用于对映选择性催化的机械平面手性轮烷配体的合成。
轮烷是互锁分子,其中分子环被困在哑铃形轴上,因为它无法从庞大的端基上逸出,从而形成所谓的机械键。互锁分子主要作为分子机器的组成部分进行研究,但在催化和传感方面也探索了通过将一个分子穿过另一个分子所产生的拥挤、灵活的环境。然而,到目前为止,互锁分子最有趣的特性之一的应用,它们展示立体单元的能力,不依赖于其共价亚基的立体化学,称为“机械手性”,尚未得到适当的探索,并且机械手性轮烷应用的原型演示仍然很少。这里,



























 京公网安备 11010802027423号
京公网安备 11010802027423号