Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2020-03-09 , DOI: 10.1016/j.bmcl.2020.127099 Ana V Cheng 1 , Cassandra L Schrank 1 , Iliana E Escobar 2 , Eleftherios Mylonakis 2 , William M Wuest 1
|
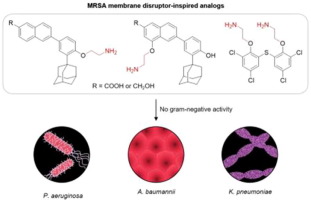
Our labs have demonstrated the activity of bithionol and synthetic retinoids against methicillin-resistant Staphylococcus aureus (MRSA), as well as their membrane-acting mechanism of action. However, the compounds lack activity in gram-negative species. Herein, we apply a known strategy for converting gram-positive agents into broad-spectrum therapies: addition of an alkylamine. By appending an alkylamine to the phenols of these known membrane disruptors, we test whether this approach is applicable to our compounds. Ultimately, biological testing in four MRSA strains and three gram-negative species showed abolished or diminished activity in all our analogs compared to their parent compounds and no gram-negative activity. Thus, we find that alkylamines would not elicit broad-spectrum activity from bithionol or CD437 derivatives.
中文翻译:

在乙二酚和合成类维生素A的苯酚中添加乙胺不会引起革兰氏阴性细菌的活性。
我们的实验室已证明了联苯酚和合成类维生素A的抗甲氧西林金黄色葡萄球菌(MRSA)的活性,以及它们的膜作用机制。但是,这些化合物在革兰氏阴性菌种中没有活性。在本文中,我们应用了将革兰氏阳性药物转化为广谱疗法的已知策略:添加烷基胺。通过将烷基胺添加到这些已知的膜破坏剂的苯酚中,我们测试了这种方法是否适用于我们的化合物。最终,在四个MRSA菌株和三个革兰氏阴性菌种中进行的生物学测试显示,与它们的母体化合物相比,我们所有类似物的活性均被消除或减弱,而革兰氏阴性则无活性。因此,我们发现烷基胺不会从联苯酚或CD437衍生物中引发广谱活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号