Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-08 , DOI: 10.1016/j.tet.2020.131111 Patrik Oleksak , Jozef Gonda , Jan Imrich , David Malinak , Antonin Lycka , Kamil Musilek
|
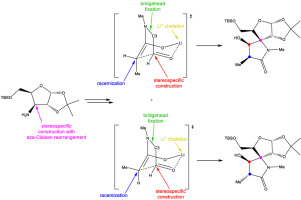
A stereoselective approach to C-3′ methyl attached γ-lactam derivatives as the precursors of pyroglutamate core of oxazolomycins is described. The synthesis started from d-xylose and utilized [3,3]-heterosigmatropic rearrangement. The key lactamization was achieved with intramolecular aldol reaction and ring-closing metathesis. The accomplished stereoselective aldol cyclization was elucidated with Zimmerman-Traxler transition state models. Stereoselectivity of the aldol reaction was explained as a consequence of chelation and bridgehead atoms linkage. The present work also encompasses proposed structures of (E)- and (Z)- generated enolates during intramolecular aldol reaction. Although stereocenters were not formed during ring-closing metathesis, a stereospecific reduction of prepared double bond functionality was successfully accomplished.
中文翻译:

立体选择法制备恶唑球霉素的γ-内酰胺前体
描述了一种立体选择性的方法,用于连接作为恶唑霉素的焦谷氨酸核心的C-3'甲基连接的γ-内酰胺衍生物。合成从d-木糖开始,并利用[3,3]-异向异构重排。关键的内酰胺化是通过分子内羟醛反应和闭环复分解实现的。Zimmerman-Traxler过渡态模型阐明了已完成的立体选择性醛醇缩合环化反应。醛醇反应的立体选择性是由于螯合和桥头原子键合的结果。本工作还涵盖(E)-和(Z)-在分子内羟醛反应中产生的烯醇化物。尽管在闭环复分解过程中未形成立体中心,但成功实现了立体定向的双键官能度降低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号