当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Behavior and Thermodynamic Modeling of Inosine (Form β) in Four Cosolvency Systems at T = 278.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-09 , DOI: 10.1021/acs.jced.0c00046 Yuli Shi 1, 2 , Haojian Zhang 1 , Xiaodong Wang 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-09 , DOI: 10.1021/acs.jced.0c00046 Yuli Shi 1, 2 , Haojian Zhang 1 , Xiaodong Wang 2
Affiliation
|
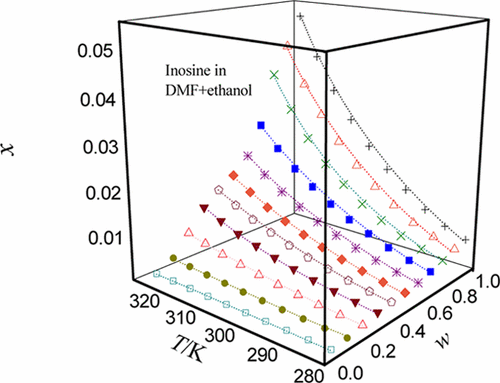
N,N-Dimethylformamide (DMF) as the main solvent with strong dissolving power was blended with four secondary solvents (ethanol, n-propanol, isopropanol, and propylene glycol) with relatively weak dissolving power to form many new solvents. The dispersion index (inosine (form β) mole fraction) in five organic solvents such as DMF and in the newly formed solvent was also obtained one by one with the static method commonly used in solid–liquid equilibrium. The temperature environment includes the high temperature set at 323.15 K, the low temperature set at 278.15 K, and an interval between each temperature of 5 K. The pressure environment was the atmospheric pressure in the natural state, and the usual value was 101.0 kPa. In a mixed system, temperature was a non-negligible influencing factor from beginning to end, and its increase often led the solute to the trend of high solubility. In addition, the proportion of the main solvent also dominated the solubilization trend of the inosine (form β); the larger the proportion of DMF, the easier the dissolution process. When both of the above factors were fixed at a certain point, the dispersing ability of the dispersing liquid composed of DMF and ethanol was undoubtedly the first. Three models (Jouyban–Acree model, van’t Hoff–Jouyban–Acree model, and modified Apelblat–Jouyban–Acree model) were used to correlate the solubility data. The largest relative average deviation and root-mean-square deviation values were 4.66 × 10–2 and 7.27 × 10–4, respectively. The dispersion data of inosine (form β) obtained through this experimental process and related thermodynamic parameters obtained through thermodynamic calculations have important application significance for its industrial production and further purification.
中文翻译:

在T = 278.15至323.15 K的情况下,在四个共混体系中肌苷(β型)的溶解度行为和热力学模型
Ñ,Ñ二甲基甲酰胺(DMF)作为具有较强的溶解力的主要溶剂具有四个第二溶剂(乙醇,混合Ñ-丙醇,异丙醇和丙二醇)的溶解力相对较弱,会形成许多新的溶剂。还使用固液平衡中常用的静态方法一一获得了在五种有机溶剂(如DMF)和新形成的溶剂中的分散指数(肌苷(β型)摩尔分数)。温度环境包括设定为323.15 K的高温,设定为278.15 K的低温以及每个温度之间的间隔5K。压力环境为自然状态下的大气压,通常值为101.0 kPa。在混合系统中,温度从始至终都是不可忽略的影响因素,温度的升高通常导致溶质趋向于高溶解度的趋势。此外,主要溶剂的比例也支配肌苷(形式β)的增溶趋势;DMF的比例越大,溶解过程越容易。当上述两个因素都固定在某个点时,由DMF和乙醇组成的分散液的分散能力无疑是第一位的。三种模型(Jouyban–Acree模型,van't Hoff–Jouyban–Acree模型和改良的Apelblat–Jouyban–Acree模型)用于关联溶解度数据。最大相对平均偏差和均方根偏差值为4.66×10 三种模型(Jouyban–Acree模型,van't Hoff–Jouyban–Acree模型和改良的Apelblat–Jouyban–Acree模型)用于关联溶解度数据。最大相对平均偏差和均方根偏差值为4.66×10 三种模型(Jouyban–Acree模型,van't Hoff–Jouyban–Acree模型和改良的Apelblat–Jouyban–Acree模型)用于关联溶解度数据。最大相对平均偏差和均方根偏差值为4.66×10–2和7.27×10 –4。通过该实验过程获得的肌苷(β型)的分散数据以及通过热力学计算获得的相关热力学参数对其工业生产和进一步纯化具有重要的应用意义。
更新日期:2020-04-24
中文翻译:

在T = 278.15至323.15 K的情况下,在四个共混体系中肌苷(β型)的溶解度行为和热力学模型
Ñ,Ñ二甲基甲酰胺(DMF)作为具有较强的溶解力的主要溶剂具有四个第二溶剂(乙醇,混合Ñ-丙醇,异丙醇和丙二醇)的溶解力相对较弱,会形成许多新的溶剂。还使用固液平衡中常用的静态方法一一获得了在五种有机溶剂(如DMF)和新形成的溶剂中的分散指数(肌苷(β型)摩尔分数)。温度环境包括设定为323.15 K的高温,设定为278.15 K的低温以及每个温度之间的间隔5K。压力环境为自然状态下的大气压,通常值为101.0 kPa。在混合系统中,温度从始至终都是不可忽略的影响因素,温度的升高通常导致溶质趋向于高溶解度的趋势。此外,主要溶剂的比例也支配肌苷(形式β)的增溶趋势;DMF的比例越大,溶解过程越容易。当上述两个因素都固定在某个点时,由DMF和乙醇组成的分散液的分散能力无疑是第一位的。三种模型(Jouyban–Acree模型,van't Hoff–Jouyban–Acree模型和改良的Apelblat–Jouyban–Acree模型)用于关联溶解度数据。最大相对平均偏差和均方根偏差值为4.66×10 三种模型(Jouyban–Acree模型,van't Hoff–Jouyban–Acree模型和改良的Apelblat–Jouyban–Acree模型)用于关联溶解度数据。最大相对平均偏差和均方根偏差值为4.66×10 三种模型(Jouyban–Acree模型,van't Hoff–Jouyban–Acree模型和改良的Apelblat–Jouyban–Acree模型)用于关联溶解度数据。最大相对平均偏差和均方根偏差值为4.66×10–2和7.27×10 –4。通过该实验过程获得的肌苷(β型)的分散数据以及通过热力学计算获得的相关热力学参数对其工业生产和进一步纯化具有重要的应用意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号