当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate Activation of the Low-Molecular Weight Protein Tyrosine Phosphatase from Mycobacterium tuberculosis
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-13 , DOI: 10.1021/acs.biochem.0c00059 Alessandra Stefan 1, 2 , Fabrizio Dal Piaz 3 , Antonio Girella 1 , Alejandro Hochkoeppler 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-13 , DOI: 10.1021/acs.biochem.0c00059 Alessandra Stefan 1, 2 , Fabrizio Dal Piaz 3 , Antonio Girella 1 , Alejandro Hochkoeppler 1, 2
Affiliation
|
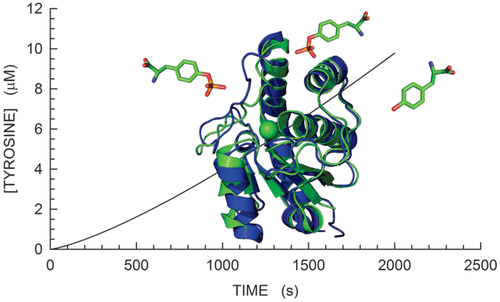
Mycobacterium tuberculosis is known to express a low-molecular weight protein tyrosine phosphatase. This enzyme, denoted as MptpA (Mycobacterium protein tyrosine phosphatase A), is essential for the pathogen to escape the host immune system and therefore represents a target for the search of antituberculosis drugs. MptpA was shown to undergo a conformational transition during catalysis, leading to the closure of the active site, which is by this means poised to the chemical step of dephosphorylation. Here we show that MptpA is subjected to substrate activation, triggered by p-nitrophenyl phosphate or by phosphotyrosine. Moreover, we show that the enzyme is also activated by phosphoserine, with serine being ineffective in enhancing MptpA activity. In addition, we performed assays under pre-steady-state conditions, and we show here that substrate activation is kinetically coupled to the closure of the active site. Surprisingly, when phosphotyrosine was used as a substrate, MptpA did not obey Michealis–Menten kinetics, but we observed a sigmoidal dependence of the reaction velocity on substrate concentration, suggesting the presence of an allosteric activating site in MptpA. This site could represent a promising target for the screening of MptpA inhibitors exerting their action independently of the binding to the enzyme active site.
中文翻译:

结核分枝杆菌低分子量蛋白酪氨酸磷酸酶的底物活化
已知结核分枝杆菌表达低分子量蛋白酪氨酸磷酸酶。这种酶称为MptpA(分枝杆菌蛋白酪氨酸磷酸酶A),对于病原体逃脱宿主免疫系统至关重要,因此代表了寻找抗结核药物的目标。已显示MptpA在催化过程中经历构象转变,从而导致活性位点的关闭,这意味着已准备好进行去磷酸化的化学步骤。在这里,我们显示MptpA受p触发的底物激活磷酸硝基苯酯或磷酸酪氨酸。此外,我们显示该酶也被磷酸丝氨酸激活,丝氨酸不能有效增强MptpA活性。另外,我们在稳态之前的条件下进行了测定,并且在此表明底物活化在动力学上与活性位点的闭合有关。出乎意料的是,当磷酸酪氨酸用作底物时,MptpA并未遵循米歇斯-门腾动力学,但我们观察到反应速度对底物浓度呈S型依赖,表明在MptpA中存在变构活化位点。该位点可以代表筛选其发挥作用的MptpA抑制剂的有前途的目标,而这些作用独立于与酶活性位点的结合。
更新日期:2020-03-16
中文翻译:

结核分枝杆菌低分子量蛋白酪氨酸磷酸酶的底物活化
已知结核分枝杆菌表达低分子量蛋白酪氨酸磷酸酶。这种酶称为MptpA(分枝杆菌蛋白酪氨酸磷酸酶A),对于病原体逃脱宿主免疫系统至关重要,因此代表了寻找抗结核药物的目标。已显示MptpA在催化过程中经历构象转变,从而导致活性位点的关闭,这意味着已准备好进行去磷酸化的化学步骤。在这里,我们显示MptpA受p触发的底物激活磷酸硝基苯酯或磷酸酪氨酸。此外,我们显示该酶也被磷酸丝氨酸激活,丝氨酸不能有效增强MptpA活性。另外,我们在稳态之前的条件下进行了测定,并且在此表明底物活化在动力学上与活性位点的闭合有关。出乎意料的是,当磷酸酪氨酸用作底物时,MptpA并未遵循米歇斯-门腾动力学,但我们观察到反应速度对底物浓度呈S型依赖,表明在MptpA中存在变构活化位点。该位点可以代表筛选其发挥作用的MptpA抑制剂的有前途的目标,而这些作用独立于与酶活性位点的结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号