当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
OH• + HCl Reaction at the Surface of a Water Droplet: An Ab Initio Molecular Dynamical Study
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-17 , DOI: 10.1021/acs.jpcb.9b11813 Subhasish Mallick 1 , Pradeep Kumar 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-03-17 , DOI: 10.1021/acs.jpcb.9b11813 Subhasish Mallick 1 , Pradeep Kumar 1
Affiliation
|
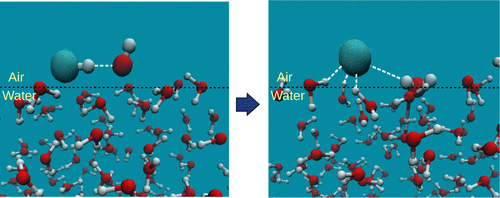
The Born–Oppenheimer molecular dynamics (BOMD) simulation has been performed to investigate the dynamics of the OH• + HCl reaction at the surface of a water droplet. The investigation suggests that the reaction occurred at the surface of the water droplet becomes almost 10 times faster than the corresponding gas-phase reaction. Besides, we have also performed the quantum mechanics/molecular mechanics calculation to calculate the unimolecular energy barrier of the reaction. The results indicate that the barrier height gets decreased by ∼0.3 kcal mol–1 at the surface of the water droplet, which also justifies the rate enhancement suggested by the BOMD simulation. The BOMD simulation also indicates that, at equilibrium, the product Cl• forms four hydrogen bonds with four interfacial water molecules, which stabilize the Cl• and resist it to escape from the surface.
中文翻译:

水滴表面的OH • + HCl反应:从头算分子动力学研究
已经进行了Born–Oppenheimer分子动力学(BOMD)模拟,以研究水滴表面OH • + HCl反应的动力学。研究表明,在水滴表面发生的反应比相应的气相反应快了近十倍。此外,我们还进行了量子力学/分子力学计算,以计算反应的单分子能垒。结果表明,在水滴表面的势垒高度降低了约0.3 kcal mol –1,这也证明了BOMD模拟所建议的速率提高是合理的。BOMD模拟还表明,在平衡时,乘积Cl •与四个界面水分子形成四个氢键,从而使Cl •稳定并阻止其从表面逸出。
更新日期:2020-03-19
中文翻译:

水滴表面的OH • + HCl反应:从头算分子动力学研究
已经进行了Born–Oppenheimer分子动力学(BOMD)模拟,以研究水滴表面OH • + HCl反应的动力学。研究表明,在水滴表面发生的反应比相应的气相反应快了近十倍。此外,我们还进行了量子力学/分子力学计算,以计算反应的单分子能垒。结果表明,在水滴表面的势垒高度降低了约0.3 kcal mol –1,这也证明了BOMD模拟所建议的速率提高是合理的。BOMD模拟还表明,在平衡时,乘积Cl •与四个界面水分子形成四个氢键,从而使Cl •稳定并阻止其从表面逸出。











































 京公网安备 11010802027423号
京公网安备 11010802027423号