当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IR Cavity Ringdown Spectroscopy and Density Functional Theory for Jet-Cooled Pyrrole–Cyclopentanone Binary Clusters: Effect of Pseudorotation on N—H···O═C Hydrogen Bonds
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-13 , DOI: 10.1021/acs.jpca.0c00794 Yoshiteru Matsumoto 1 , Kenji Honma 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-13 , DOI: 10.1021/acs.jpca.0c00794 Yoshiteru Matsumoto 1 , Kenji Honma 2
Affiliation
|
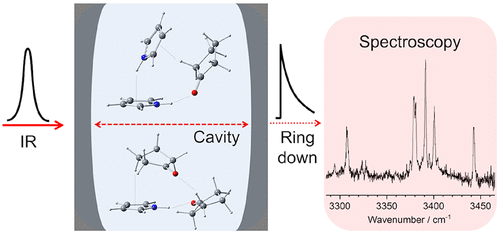
The geometry and energetics of the N—H···O═C hydrogen bond (H-bond) are important to understand the stability and flexibility of biomolecules, such as protein and DNA. Jet-cooled pyrrole–cyclopentanone (Py–Cp) binary clusters are appropriate models to investigate the N—H···O═C H-bond from a microscopic point of view. In this study, NH stretching vibrations of the Py–Cp binary clusters were observed by IR cavity ringdown spectroscopy. Furthermore, density functional theory calculations revealed geometric structures, harmonic vibrations, intermolecular energies, and donor–acceptor interactions for various sizes of binary clusters. The IR spectra of the Py–Cp binary clusters were measured under various conditions of the vapor pressures of Py and Cp in He buffer gas for a supersonic expansion. The dependence of the IR band intensities on the vapor pressure provides vibrational assignments of the NH stretching vibrations, which were reproduced by calculated frequencies of Py1–Cp1, Py1–Cp2, and Py2–Cp1. An admixture of Ar in He buffer gas for a supersonic expansion was also applied to produce Py1–Cp2 in order to differentiate several NH stretches of isomeric structures due to the pseudorotation of Cp molecules. Py1–Cp1 is formed by the N—H···O═C H-bond. Py1–Cp2 has a cyclic structure that is formed by the N—H···O═C H-bond and stacking interactions among Py and two Cp molecules. Py2–Cp1 also has a cyclic structure that is formed by not only the N—H···O═C H-bond but also a N—H···π H-bond between two Py molecules and a stacking interaction between Py and Cp. A comparison of the H-bond geometries between Py2–Cp1 and the corresponding pyrrole–acetone binary cluster reveals that the stacking interaction between Py and Cp strengthens the N—H···O═C H-bond through a cooperative effect.
中文翻译:

射流冷却的吡咯-环戊烷酮二元团簇的红外腔衰荡光谱和密度泛函理论:伪旋转对NH···O═C氢键的影响
N-H···O═C氢键(H键)的几何结构和高能学对于理解蛋白质和DNA等生物分子的稳定性和灵活性很重要。射流冷却的吡咯-环戊酮(Py-Cp)二元簇是从微观角度研究NH···O═CH键的合适模型。在这项研究中,通过红外腔衰荡光谱观察了Py–Cp二元簇的NH拉伸振动。此外,密度泛函理论的计算揭示了各种大小的二元簇的几何结构,谐波振动,分子间能量以及供体-受体相互作用。在氦气缓冲气体中Py和Cp的蒸气压发生超音速膨胀的各种条件下,测量了Py–Cp二元簇的红外光谱。1 -Cp 1,Py 1 -Cp 2和Py 2 -Cp 1。为了区分由于Cp分子的伪旋转引起的几个NH异构体结构的延伸,还应用了He缓冲气体中的Ar混合物进行超音速膨胀,以产生Py 1 -Cp 2。Py 1 -Cp 1由N·H··O═CH键形成。Py 1 –Cp 2具有由NH···O═CH键和Py与两个Cp分子之间的堆叠相互作用形成的环状结构。Py 2 –Cp 1还具有环状结构,该环状结构不仅由两个Py分子之间的NH-··O HC H键而且由NH···πH键以及Py和Cp之间的堆积相互作用形成。对Py 2 -Cp 1和相应的吡咯-丙酮二元簇之间的H键几何结构进行比较,发现Py和Cp之间的堆积相互作用通过协同效应增强了NH···O═CH键。
更新日期:2020-03-16
中文翻译:

射流冷却的吡咯-环戊烷酮二元团簇的红外腔衰荡光谱和密度泛函理论:伪旋转对NH···O═C氢键的影响
N-H···O═C氢键(H键)的几何结构和高能学对于理解蛋白质和DNA等生物分子的稳定性和灵活性很重要。射流冷却的吡咯-环戊酮(Py-Cp)二元簇是从微观角度研究NH···O═CH键的合适模型。在这项研究中,通过红外腔衰荡光谱观察了Py–Cp二元簇的NH拉伸振动。此外,密度泛函理论的计算揭示了各种大小的二元簇的几何结构,谐波振动,分子间能量以及供体-受体相互作用。在氦气缓冲气体中Py和Cp的蒸气压发生超音速膨胀的各种条件下,测量了Py–Cp二元簇的红外光谱。1 -Cp 1,Py 1 -Cp 2和Py 2 -Cp 1。为了区分由于Cp分子的伪旋转引起的几个NH异构体结构的延伸,还应用了He缓冲气体中的Ar混合物进行超音速膨胀,以产生Py 1 -Cp 2。Py 1 -Cp 1由N·H··O═CH键形成。Py 1 –Cp 2具有由NH···O═CH键和Py与两个Cp分子之间的堆叠相互作用形成的环状结构。Py 2 –Cp 1还具有环状结构,该环状结构不仅由两个Py分子之间的NH-··O HC H键而且由NH···πH键以及Py和Cp之间的堆积相互作用形成。对Py 2 -Cp 1和相应的吡咯-丙酮二元簇之间的H键几何结构进行比较,发现Py和Cp之间的堆积相互作用通过协同效应增强了NH···O═CH键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号