当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent Effects on Thiol–Ene Kinetics and Reactivity of Carbon and Sulfur Radicals
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jpca.9b10165 Ipek Munar 1 , Volkan Fındık 2 , Isa Degirmenci 3 , Viktorya Aviyente 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.jpca.9b10165 Ipek Munar 1 , Volkan Fındık 2 , Isa Degirmenci 3 , Viktorya Aviyente 1
Affiliation
|
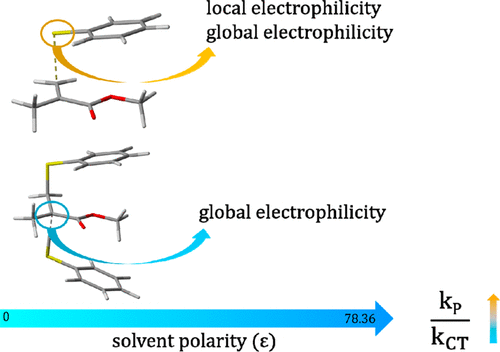
The thiol–ene reaction is one of the fundamental reactions in biochemistry and synthetic organic chemistry. In this study, the effect of polar media on the reaction kinetics is taken into account by using the transition state theory; the reactivities of the carbon and sulfur radicals have also been rationalized by using conceptual DFT. The results have shown that the solvents have more impact on hydrogen atom transfer reactions and the chain transfer rate constant, kCT, can be increased by using nonpolar solvents, while propagation reactions are less sensitive to media. Similarly, the kP/kCT ratio can be manipulated by changing the environment in order to obtain tailor-made polymers. Regarding the DFT descriptors, the local and global electrophilicity indices are well correlated with the propagation rate constant kP, whereas the global electrophilicity index is associated with the chain transfer rate constant kCT. Overall, electrophilicity indices can be used with confidence to predict the kinetics of thiol–ene reactions
中文翻译:

溶剂对硫醇动力学及碳和硫自由基反应性的影响
硫醇-烯反应是生物化学和合成有机化学中的基本反应之一。在这项研究中,使用过渡态理论考虑了极性介质对反应动力学的影响。碳和硫自由基的反应性也已通过使用概念DFT得到合理化。结果表明,溶剂对氢原子转移反应的影响更大,通过使用非极性溶剂可以增加链转移速率常数k CT,而传播反应对介质的敏感性较低。同样,k P / k CT可以通过改变环境来控制比例,以获得定制的聚合物。关于DFT描述符,局部和全局亲电指数与传播速率常数k P良好相关,而全局亲电指数与链转移速率常数k CT相关。总体而言,亲电指数可用于预测硫醇-烯反应的动力学
更新日期:2020-03-21
中文翻译:

溶剂对硫醇动力学及碳和硫自由基反应性的影响
硫醇-烯反应是生物化学和合成有机化学中的基本反应之一。在这项研究中,使用过渡态理论考虑了极性介质对反应动力学的影响。碳和硫自由基的反应性也已通过使用概念DFT得到合理化。结果表明,溶剂对氢原子转移反应的影响更大,通过使用非极性溶剂可以增加链转移速率常数k CT,而传播反应对介质的敏感性较低。同样,k P / k CT可以通过改变环境来控制比例,以获得定制的聚合物。关于DFT描述符,局部和全局亲电指数与传播速率常数k P良好相关,而全局亲电指数与链转移速率常数k CT相关。总体而言,亲电指数可用于预测硫醇-烯反应的动力学











































 京公网安备 11010802027423号
京公网安备 11010802027423号