当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Reduction of Oxazolyl‐Bearing Secondary Anilides to Amines by Nickel‐Catalyzed Hydrosilylation
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-19 , DOI: 10.1002/ajoc.202000054 Jian Zhou 1 , Xiaofan Bo 1 , Li Wan 1 , Zhong Xin 1, 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-19 , DOI: 10.1002/ajoc.202000054 Jian Zhou 1 , Xiaofan Bo 1 , Li Wan 1 , Zhong Xin 1, 2
Affiliation
|
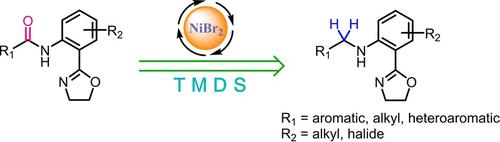
A convenient procedure for the catalytic hydrosilylation of secondary anilides bearing an oxazolyl group has been developed. In the presence of inexpensive NiBr2, the reduction of functionalized secondary amides with nontoxic and air‐stable TMDS proceeded smoothly to give the corresponding functionalized amines. The method displays a broad applicability for the reduction of many types of substrates, and shows good compatibility and excellent chemoselectivity for several sensitive functional groups such as halo, ester and nitro groups.
中文翻译:

镍催化的氢化硅烷化将含恶唑基的仲酸酐有效还原为胺
已经开发了一种方便的方法,用于带有恶唑基的仲酸酐催化氢化硅烷化。在廉价的NiBr 2存在下,用无毒且空气稳定的TMDS还原官能化仲酰胺可顺利进行,得到相应的官能化胺。该方法对减少多种类型的底物显示出广泛的适用性,并且对几种敏感的官能团如卤素,酯和硝基具有良好的相容性和优异的化学选择性。
更新日期:2020-03-19
中文翻译:

镍催化的氢化硅烷化将含恶唑基的仲酸酐有效还原为胺
已经开发了一种方便的方法,用于带有恶唑基的仲酸酐催化氢化硅烷化。在廉价的NiBr 2存在下,用无毒且空气稳定的TMDS还原官能化仲酰胺可顺利进行,得到相应的官能化胺。该方法对减少多种类型的底物显示出广泛的适用性,并且对几种敏感的官能团如卤素,酯和硝基具有良好的相容性和优异的化学选择性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号