当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Ligand Fields on the Reactivity of O2 -Activating Iron(II)-Benzilate Complexes of Neutral N5 Donor Ligands.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-03-24 , DOI: 10.1002/asia.202000142 Shrabanti Bhattacharya 1 , Reena Singh 1 , Tapan Kanti Paine 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-03-24 , DOI: 10.1002/asia.202000142 Shrabanti Bhattacharya 1 , Reena Singh 1 , Tapan Kanti Paine 1
Affiliation
|
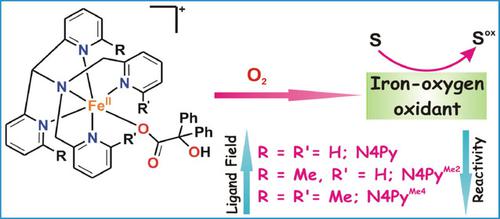
Three new iron(II)-benzilate complexes [(N4Py)FeII (benzilate)]ClO4 (1), [(N4PyMe2 )FeII (benzilate)]ClO4 (2) and [(N4PyMe4 )FeII (benzilate)]ClO4 (3) of neutral pentadentate nitrogen donor ligands have been isolated and characterized to study their dioxygen reactivity. Single-crystal X-ray structures reveal a mononuclear six-coordinate iron(II) center in each case, where benzilate binds to the iron center in monodentate mode via one carboxylate oxygen. Introduction of methyl groups in the 6-positions of the pyridine rings makes the N4PyMe2 and N4PyMe4 ligand fields weaker compared to that of the parent N4Py ligand. All the complexes (1-3) react with dioxygen to decarboxylate the coordinated benzilate to benzophenone quantitatively. The decarboxylation is faster for the complex of the more sterically hindered ligand and follows the order 3>2>1. The complexes display oxygen atom transfer reactivity to thioanisole and also exhibit hydrogen atom transfer reactions with substrates containing weak C-H bonds. Based on interception studies with external substrates, labelling experiments and Hammett analysis, a nucleophilic iron(II)-hydroperoxo species is proposed to form upon two-electron reductive activation of dioxygen by each iron(II)-benzilate complex. The nucleophilic oxidants are converted to the corresponding electrophilic iron(IV)-oxo oxidant upon treatment with a protic acid. The high-spin iron(II)-benzilate complex with the weakest ligand field results in the formation of a more reactive iron-oxygen oxidant.
中文翻译:

配体场对中性N5供体配体的O2活化铁(II)-苯甲酸酯配合物反应性的影响。
三种新的铁(II)-苯甲酸酯络合物[(N4Py)FeII(苯甲酸酯)] ClO4(1),[(N4PyMe2)FeII(苯甲酸酯)] ClO4(2)和[(N4PyMe4)FeII(苯甲酸酯)] ClO4(3)已分离出中性的五齿氮供体配体,并对其特征进行了研究以研究其双氧反应性。单晶X射线结构在每种情况下都显示一个单核六坐标铁(II)中心,其中苯甲酸酯通过一个羧酸氧以单齿模式结合到铁中心。与母体N4Py配体相比,在吡啶环的6位引入甲基使得N4PyMe2和N4PyMe4配体场更弱。所有的配合物(1-3)与双氧反应以将配位的苯甲酸酯定量地脱羧为二苯甲酮。对于位阻更强的配体的配合物,脱羧速度更快,并且遵循3> 2>的顺序 1.配合物显示出与硫代苯甲醚的氧原子转移反应性,并且还与含有弱CH键的底物发生氢原子转移反应。基于与外部底物的拦截研究,标记实验和Hammett分析,提出了一种亲核性铁(II)-氢过氧物质,可通过每个铁(II)-苯甲酸酯络合物的双电子双氧还原活化而形成。用质子酸处理后,亲核氧化剂被转化为相应的亲电子氧化铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。基于与外部底物的拦截研究,标记实验和Hammett分析,提出了一种亲核性铁(II)-氢过氧物质,可通过每个铁(II)-苯甲酸酯络合物的双电子双氧还原活化而形成。用质子酸处理后,亲核氧化剂被转化为相应的亲电铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。基于与外部底物的拦截研究,标记实验和Hammett分析,提出了一种亲核性铁(II)-氢过氧物质,可通过每个铁(II)-苯甲酸酯络合物的双电子双氧还原活化而形成。用质子酸处理后,亲核氧化剂被转化为相应的亲电子氧化铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。用质子酸处理后,亲核氧化剂被转化为相应的亲电铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。用质子酸处理后,亲核氧化剂被转化为相应的亲电铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。
更新日期:2020-04-22
中文翻译:

配体场对中性N5供体配体的O2活化铁(II)-苯甲酸酯配合物反应性的影响。
三种新的铁(II)-苯甲酸酯络合物[(N4Py)FeII(苯甲酸酯)] ClO4(1),[(N4PyMe2)FeII(苯甲酸酯)] ClO4(2)和[(N4PyMe4)FeII(苯甲酸酯)] ClO4(3)已分离出中性的五齿氮供体配体,并对其特征进行了研究以研究其双氧反应性。单晶X射线结构在每种情况下都显示一个单核六坐标铁(II)中心,其中苯甲酸酯通过一个羧酸氧以单齿模式结合到铁中心。与母体N4Py配体相比,在吡啶环的6位引入甲基使得N4PyMe2和N4PyMe4配体场更弱。所有的配合物(1-3)与双氧反应以将配位的苯甲酸酯定量地脱羧为二苯甲酮。对于位阻更强的配体的配合物,脱羧速度更快,并且遵循3> 2>的顺序 1.配合物显示出与硫代苯甲醚的氧原子转移反应性,并且还与含有弱CH键的底物发生氢原子转移反应。基于与外部底物的拦截研究,标记实验和Hammett分析,提出了一种亲核性铁(II)-氢过氧物质,可通过每个铁(II)-苯甲酸酯络合物的双电子双氧还原活化而形成。用质子酸处理后,亲核氧化剂被转化为相应的亲电子氧化铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。基于与外部底物的拦截研究,标记实验和Hammett分析,提出了一种亲核性铁(II)-氢过氧物质,可通过每个铁(II)-苯甲酸酯络合物的双电子双氧还原活化而形成。用质子酸处理后,亲核氧化剂被转化为相应的亲电铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。基于与外部底物的拦截研究,标记实验和Hammett分析,提出了一种亲核性铁(II)-氢过氧物质,可通过每个铁(II)-苯甲酸酯络合物的双电子双氧还原活化而形成。用质子酸处理后,亲核氧化剂被转化为相应的亲电子氧化铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。用质子酸处理后,亲核氧化剂被转化为相应的亲电铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。用质子酸处理后,亲核氧化剂被转化为相应的亲电铁(IV)-氧代氧化剂。具有最弱配体场的高自旋铁(II)-苯甲酸酯络合物导致形成更具反应性的铁-氧氧化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号