当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
5‐[2‐(N‐(Substituted phenyl)acetamide)]amino‐1,3,4‐thiadiazole‐2‐sulfonamides as Selective Carbonic Anhydrase II Inhibitors with Neuroprotective Effects
ChemMedChem ( IF 3.6 ) Pub Date : 2020-03-18 , DOI: 10.1002/cmdc.201900703 Caibao Jiang 1 , Jinguo Shi 1 , Liping Liao 1 , Liantao Zhang 1 , Jiayong Liu 1 , Yang Wang 1 , Yaoqiang Lao 1 , Jingxia Zhang 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-03-18 , DOI: 10.1002/cmdc.201900703 Caibao Jiang 1 , Jinguo Shi 1 , Liping Liao 1 , Liantao Zhang 1 , Jiayong Liu 1 , Yang Wang 1 , Yaoqiang Lao 1 , Jingxia Zhang 1
Affiliation
|
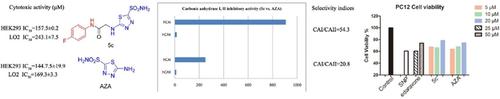
In this study, 22 novel compounds were designed and synthesized by acetamide bridge chains, among which 5 a–5 k were monosubstituted compounds, and 6 a–6 k were disubstituted. A series of biological evaluations was then carried out to determine the carbonic anhydrase inhibitory activity, neuroprotective effects and cytotoxicity of 5 a–5 k and 6 a–6 k. The results showed that some compounds could protect PC12 cells from sodium nitroprusside (SNP)‐induced damage. In terms of the neuroprotection and inhibitory activity against carbonic anhydrase II, monosubstituted compounds were better than disubstituted. Compound 5 c exhibited better protective effect in PC12 cells than that of edaravone, and 5 c also showed less cytotoxicity. In addition, compound 5 c was found to be the most effective selective carbonic anhydrase II inhibitor (IC50=16.7 nM, CAI/CAII=54.3), which was similar to the inhibitory effect of acetazolamide. Moreover, the selectivity of compound 5 c was better than that of acetazolamide (IC50=12.0 nM, CAI/CAII=20.8). Molecular docking presented that the binding effect of compound 5 c with carbonic anhydrase II was superior to that of 5 c with carbonic anhydrase I and IX, which was consistent with the inhibitory results. Based on above findings, compound 5 c may be a potential candidate for selective carbonic anhydrase II inhibitor, and it had obviously neuroprotective effect and great advantages in drug safety.
中文翻译:

5- [2-(N-(取代苯基)乙酰胺)]氨基-1,3,4-噻二唑-2-磺酰胺作为选择性碳酸酐酶II抑制剂,具有神经保护作用
在这项研究中,通过乙酰胺桥链设计和合成了22种新型化合物,其中5 a - 5 k是单取代的化合物,6 a - 6 k是双取代的。然后进行了一系列生物学评估,以确定5 a – 5 k和6 a – 6 k的碳酸酐酶抑制活性,神经保护作用和细胞毒性。结果表明,某些化合物可以保护PC12细胞免受硝普钠(SNP)诱导的损害。就对碳酸酐酶II的神经保护和抑制活性而言,单取代的化合物优于二取代的化合物。化合物5 c在PC12细胞中显示出比依达拉奉更好的保护作用,而5 c也显示出较小的细胞毒性。另外,发现化合物5c是最有效的选择性碳酸酐酶II抑制剂(IC 50= 16.7nM,CAI / CAII = 54.3),其与乙酰唑胺的抑制作用相似。此外,化合物5c的选择性优于乙酰唑酰胺(IC 50= 12.0nM,CAI / CAII = 20.8)。分子对接表明,化合物5 c与碳酸酐酶II的结合效果优于5 c与碳酸酐酶I和IX的结合效果,这与抑制结果一致。根据以上发现,化合物5 c可能是选择性碳酸酐酶II抑制剂的潜在候选者,它具有明显的神经保护作用,在药物安全性方面具有很大的优势。
更新日期:2020-04-22
中文翻译:

5- [2-(N-(取代苯基)乙酰胺)]氨基-1,3,4-噻二唑-2-磺酰胺作为选择性碳酸酐酶II抑制剂,具有神经保护作用
在这项研究中,通过乙酰胺桥链设计和合成了22种新型化合物,其中5 a - 5 k是单取代的化合物,6 a - 6 k是双取代的。然后进行了一系列生物学评估,以确定5 a – 5 k和6 a – 6 k的碳酸酐酶抑制活性,神经保护作用和细胞毒性。结果表明,某些化合物可以保护PC12细胞免受硝普钠(SNP)诱导的损害。就对碳酸酐酶II的神经保护和抑制活性而言,单取代的化合物优于二取代的化合物。化合物5 c在PC12细胞中显示出比依达拉奉更好的保护作用,而5 c也显示出较小的细胞毒性。另外,发现化合物5c是最有效的选择性碳酸酐酶II抑制剂(IC 50= 16.7nM,CAI / CAII = 54.3),其与乙酰唑胺的抑制作用相似。此外,化合物5c的选择性优于乙酰唑酰胺(IC 50= 12.0nM,CAI / CAII = 20.8)。分子对接表明,化合物5 c与碳酸酐酶II的结合效果优于5 c与碳酸酐酶I和IX的结合效果,这与抑制结果一致。根据以上发现,化合物5 c可能是选择性碳酸酐酶II抑制剂的潜在候选者,它具有明显的神经保护作用,在药物安全性方面具有很大的优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号