Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single‐Molecule Manipulation in Zero‐Mode Waveguides
Small ( IF 13.0 ) Pub Date : 2020-03-05 , DOI: 10.1002/smll.201906740 Leonard C. Schendel 1 , Magnus S. Bauer 1 , Steffen M. Sedlak 1 , Hermann E. Gaub 1
Small ( IF 13.0 ) Pub Date : 2020-03-05 , DOI: 10.1002/smll.201906740 Leonard C. Schendel 1 , Magnus S. Bauer 1 , Steffen M. Sedlak 1 , Hermann E. Gaub 1
Affiliation
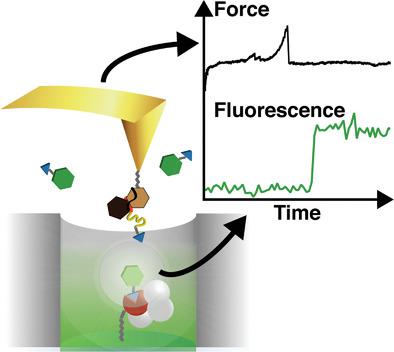
|
The mechanobiology of receptor–ligand interactions and force‐induced enzymatic turnover can be revealed by simultaneous measurements of force response and fluorescence. Investigations at physiologically relevant high labeled substrate concentrations require total internal reflection fluorescence microscopy or zero mode waveguides (ZMWs), which are difficult to combine with atomic force microscopy (AFM). A fully automatized workflow is established to manipulate single molecules inside ZMWs autonomously with noninvasive cantilever tip localization. A protein model system comprising a receptor–ligand pair of streptavidin blocked with a biotin‐tagged ligand is introduced. The ligand is pulled out of streptavidin by an AFM cantilever leaving the receptor vacant for reoccupation by freely diffusing fluorescently labeled biotin, which can be detected in single‐molecule fluorescence concurrently to study rebinding rates. This work illustrates the potential of the seamless fusion of these two powerful single‐molecule techniques.
中文翻译:

零模波导中的单分子操纵
受体-配体相互作用和力诱导的酶转化的力学生物学可以通过同时测量力响应和荧光来揭示。在生理相关的高标记底物浓度下进行的研究需要全内反射荧光显微镜或零模波导(ZMW),很难与原子力显微镜(AFM)结合使用。建立了一个完全自动化的工作流程,以通过无创悬臂尖端定位自动操纵ZMW内部的单个分子。引入了一个蛋白质模型系统,该系统包括被生物素标记的配体封闭的链霉亲和素的受体-配体对。通过AFM悬臂将配体从抗生蛋白链菌素中抽出,通过自由扩散荧光标记的生物素使受体空置以重新占据,可以同时在单分子荧光中检测到,以研究重新结合率。这项工作说明了这两种强大的单分子技术无缝融合的潜力。
更新日期:2020-04-03
中文翻译:

零模波导中的单分子操纵
受体-配体相互作用和力诱导的酶转化的力学生物学可以通过同时测量力响应和荧光来揭示。在生理相关的高标记底物浓度下进行的研究需要全内反射荧光显微镜或零模波导(ZMW),很难与原子力显微镜(AFM)结合使用。建立了一个完全自动化的工作流程,以通过无创悬臂尖端定位自动操纵ZMW内部的单个分子。引入了一个蛋白质模型系统,该系统包括被生物素标记的配体封闭的链霉亲和素的受体-配体对。通过AFM悬臂将配体从抗生蛋白链菌素中抽出,通过自由扩散荧光标记的生物素使受体空置以重新占据,可以同时在单分子荧光中检测到,以研究重新结合率。这项工作说明了这两种强大的单分子技术无缝融合的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号