当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of propargylamines via the A3 multicomponent reaction and their biological evaluation as potential anticancer agents
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/06 , DOI: 10.1039/d0ob00280a Maitena Martinez-Amezaga 1, 2, 3, 4, 5 , Rocío A. Giordano 3, 4, 5, 6, 7 , Denis N. Prada Gori 1, 2, 3, 4, 5 , Caterina Permingeat Squizatto 1, 2, 3, 4, 5 , María V. Giolito 3, 4, 5, 6, 7 , O. Graciela Scharovsky 3, 4, 5, 6, 7 , Viviana R. Rozados 3, 4, 5, 6, 7 , María J. Rico 3, 4, 5, 6, 7 , Ernesto G. Mata 1, 2, 3, 4, 5 , Carina M. L. Delpiccolo 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/06 , DOI: 10.1039/d0ob00280a Maitena Martinez-Amezaga 1, 2, 3, 4, 5 , Rocío A. Giordano 3, 4, 5, 6, 7 , Denis N. Prada Gori 1, 2, 3, 4, 5 , Caterina Permingeat Squizatto 1, 2, 3, 4, 5 , María V. Giolito 3, 4, 5, 6, 7 , O. Graciela Scharovsky 3, 4, 5, 6, 7 , Viviana R. Rozados 3, 4, 5, 6, 7 , María J. Rico 3, 4, 5, 6, 7 , Ernesto G. Mata 1, 2, 3, 4, 5 , Carina M. L. Delpiccolo 1, 2, 3, 4, 5
Affiliation
|
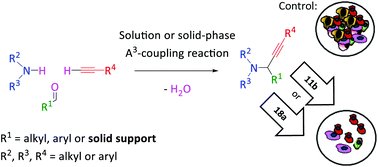
Propargylamines have gained importance in the area of anticancer research. We synthesized 1-substituted propargylic tertiary amines using the A3-coupling as the key step. Both, solution and solid-phase protocols, were used to provide a library of 1-substituted propargylic tertiary amines with interesting structural diversity. The triple negative breast cancer subtype is the most aggressive and it lacks effective therapeutic options, while pancreatic cancer is one of the neoplasms with worse prognosis and limited therapeutic possibilities. The development of tumor-selective drugs has always been a major challenge in cancer treatment. From our library, two propargylamines displayed a high degree of cytotoxic selectivity. These levels of selectivity give a very interesting perspective for further development of 1-substituted propargylic tertiary amines as new potential chemotherapeutic antitumor agents.
中文翻译:

通过A3多组分反应合成炔丙胺及其作为潜在抗癌药的生物学评估
炔丙胺在抗癌研究领域已变得越来越重要。我们使用A 3合成了1-取代的炔丙基叔胺耦合是关键步骤。溶液和固相方案均用于提供具有令人感兴趣的结构多样性的1-取代的炔丙基叔胺文库。三阴性乳腺癌亚型是最具攻击性的,缺乏有效的治疗选择,而胰腺癌是预后较差且治疗可能性有限的肿瘤之一。肿瘤选择性药物的开发一直是癌症治疗中的主要挑战。从我们的文库中,两种炔丙基胺显示出高度的细胞毒性选择性。这些选择性水平为1-取代的炔丙基叔胺作为新的潜在化疗抗肿瘤剂的进一步开发提供了非常有趣的前景。
更新日期:2020-04-01
中文翻译:

通过A3多组分反应合成炔丙胺及其作为潜在抗癌药的生物学评估
炔丙胺在抗癌研究领域已变得越来越重要。我们使用A 3合成了1-取代的炔丙基叔胺耦合是关键步骤。溶液和固相方案均用于提供具有令人感兴趣的结构多样性的1-取代的炔丙基叔胺文库。三阴性乳腺癌亚型是最具攻击性的,缺乏有效的治疗选择,而胰腺癌是预后较差且治疗可能性有限的肿瘤之一。肿瘤选择性药物的开发一直是癌症治疗中的主要挑战。从我们的文库中,两种炔丙基胺显示出高度的细胞毒性选择性。这些选择性水平为1-取代的炔丙基叔胺作为新的潜在化疗抗肿瘤剂的进一步开发提供了非常有趣的前景。










































 京公网安备 11010802027423号
京公网安备 11010802027423号