当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the relationship between structure and activity in BODIPYs designed for antimicrobial phototherapy
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/06 , DOI: 10.1039/d0ob00188k Benjamin F. Hohlfeld 1, 2, 3, 4, 5 , Burkhard Gitter 4, 6, 7 , Keith J. Flanagan 8, 9, 10, 11, 12 , Christopher J. Kingsbury 8, 9, 10, 11, 12 , Nora Kulak 2, 3, 4, 5, 13 , Mathias O. Senge 8, 9, 10, 11, 12 , Arno Wiehe 1, 2, 3, 4, 6
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/06 , DOI: 10.1039/d0ob00188k Benjamin F. Hohlfeld 1, 2, 3, 4, 5 , Burkhard Gitter 4, 6, 7 , Keith J. Flanagan 8, 9, 10, 11, 12 , Christopher J. Kingsbury 8, 9, 10, 11, 12 , Nora Kulak 2, 3, 4, 5, 13 , Mathias O. Senge 8, 9, 10, 11, 12 , Arno Wiehe 1, 2, 3, 4, 6
Affiliation
|
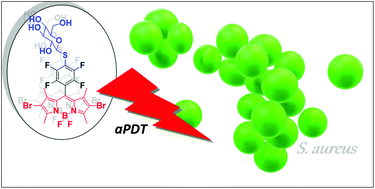
A synthetic strategy to BODIPY dyes is presented giving access to a range of new compounds relevant in the context of antimicrobial photodynamic therapy (aPDT). BODIPYs with the 8-(4-fluoro-3-nitrophenyl) and the 8-pentafluorophenyl substituents were used for the synthesis of new mono- and dibrominated BODIPYs. The para-fluorine atoms in these electron-withdrawing groups facilitate functional modification via nucleophilic aromatic substitution (SNAr) with a number of amines and thio-carbohydrates. Subsequently, the antibacterial phototoxic activity of these BODIPYs has been assessed in bacterial assays against the Gram-positive germ S. aureus and also against the Gram-negative germ P. aeruginosa. The bacterial assays allowed to identify substitution patterns which ensured antibacterial activity not only in phosphate-buffered saline (PBS) but also in the presence of serum, hereby more realistically modelling the complex biological environment that is present in clinical applications.
中文翻译:

探索用于抗菌光疗的BODIPY的结构与活性之间的关系
提出了BODIPY染料的合成策略,可提供与抗菌光动力疗法(aPDT)相关的一系列新化合物。具有8-(4-氟-3-硝基苯基)和8-五氟苯基取代基的BODIPY用于合成新的单溴和二溴BODIPY。的对位在这些吸电子基团-氟原子促进功能修饰经由亲核芳族取代(S Ñ AR)与一些胺和硫代糖类。随后,已在细菌测定中评估了这些BODIPY的抗细菌光毒性活性,这些细菌对革兰氏阳性菌金黄色葡萄球菌以及革兰氏阴性菌铜绿假单胞菌。细菌测定允许鉴定不仅在磷酸盐缓冲盐水(PBS)中而且在血清存在下均确保抗菌活性的取代模式,从而更现实地模拟临床应用中存在的复杂生物环境。
更新日期:2020-04-01
中文翻译:

探索用于抗菌光疗的BODIPY的结构与活性之间的关系
提出了BODIPY染料的合成策略,可提供与抗菌光动力疗法(aPDT)相关的一系列新化合物。具有8-(4-氟-3-硝基苯基)和8-五氟苯基取代基的BODIPY用于合成新的单溴和二溴BODIPY。的对位在这些吸电子基团-氟原子促进功能修饰经由亲核芳族取代(S Ñ AR)与一些胺和硫代糖类。随后,已在细菌测定中评估了这些BODIPY的抗细菌光毒性活性,这些细菌对革兰氏阳性菌金黄色葡萄球菌以及革兰氏阴性菌铜绿假单胞菌。细菌测定允许鉴定不仅在磷酸盐缓冲盐水(PBS)中而且在血清存在下均确保抗菌活性的取代模式,从而更现实地模拟临床应用中存在的复杂生物环境。











































 京公网安备 11010802027423号
京公网安备 11010802027423号