当前位置:
X-MOL 学术
›
PLOS Pathog.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Monocyte metabolic reprogramming promotes pro-inflammatory activity and Staphylococcus aureus biofilm clearance.
PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-03-06 , DOI: 10.1371/journal.ppat.1008354 Kelsey J Yamada 1 , Cortney E Heim 1 , Xinyuan Xi 2 , Kuldeep S Attri 3 , Dezhen Wang 3 , Wenting Zhang 2 , Pankaj K Singh 3 , Tatiana K Bronich 2 , Tammy Kielian 1
PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-03-06 , DOI: 10.1371/journal.ppat.1008354 Kelsey J Yamada 1 , Cortney E Heim 1 , Xinyuan Xi 2 , Kuldeep S Attri 3 , Dezhen Wang 3 , Wenting Zhang 2 , Pankaj K Singh 3 , Tatiana K Bronich 2 , Tammy Kielian 1
Affiliation
|
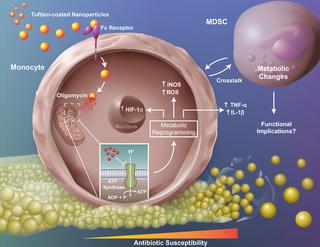
Biofilm-associated prosthetic joint infections (PJIs) cause significant morbidity due to their recalcitrance to immune-mediated clearance and antibiotics, with Staphylococcus aureus (S. aureus) among the most prevalent pathogens. We previously demonstrated that S. aureus biofilm-associated monocytes are polarized to an anti-inflammatory phenotype and the adoptive transfer of pro-inflammatory macrophages attenuated biofilm burden, highlighting the critical role of monocyte/macrophage inflammatory status in dictating biofilm persistence. The inflammatory properties of leukocytes are linked to their metabolic state, and here we demonstrate that biofilm-associated monocytes exhibit a metabolic bias favoring oxidative phosphorylation (OxPhos) and less aerobic glycolysis to facilitate their anti-inflammatory activity and biofilm persistence. To shift monocyte metabolism in vivo and reprogram cells to a pro-inflammatory state, a nanoparticle approach was utilized to deliver the OxPhos inhibitor oligomycin to monocytes. Using a mouse model of S. aureus PJI, oligomycin nanoparticles were preferentially internalized by monocytes, which significantly reduced S. aureus biofilm burden by altering metabolism and promoting the pro-inflammatory properties of infiltrating monocytes as revealed by metabolomics and RT-qPCR, respectively. Injection of oligomycin alone had no effect on monocyte metabolism or biofilm burden, establishing that intracellular delivery of oligomycin is required to reprogram monocyte metabolic activity and that oligomycin lacks antibacterial activity against S. aureus biofilms. Remarkably, monocyte metabolic reprogramming with oligomycin nanoparticles was effective at clearing established biofilms in combination with systemic antibiotics. These findings suggest that metabolic reprogramming of biofilm-associated monocytes may represent a novel therapeutic approach for PJI.
中文翻译:

单核细胞代谢重编程促进促炎活性和金黄色葡萄球菌生物膜清除。
生物膜相关的假体关节感染(PJI)由于不耐受免疫介导的清除和抗生素而导致显着的发病率,其中金黄色葡萄球菌(S. aureus)是最常见的病原体之一。我们之前证明,金黄色葡萄球菌生物膜相关单核细胞被极化为抗炎表型,促炎巨噬细胞的过继转移减轻了生物膜负担,强调了单核细胞/巨噬细胞炎症状态在决定生物膜持久性中的关键作用。白细胞的炎症特性与其代谢状态相关,在这里我们证明生物膜相关单核细胞表现出有利于氧化磷酸化(OxPhos)和较少有氧糖酵解的代谢偏差,以促进其抗炎活性和生物膜持久性。为了改变单核细胞体内代谢并将细胞重新编程至促炎状态,利用纳米颗粒方法将 OxPhos 抑制剂寡霉素递送至单核细胞。使用金黄色葡萄球菌 PJI 小鼠模型,寡霉素纳米颗粒优先被单核细胞内化,代谢组学和 RT-qPCR 分别显示,寡霉素纳米颗粒通过改变代谢和促进浸润单核细胞的促炎特性,显着减少金黄色葡萄球菌生物膜负荷。单独注射寡霉素对单核细胞代谢或生物膜负荷没有影响,这表明需要细胞内递送寡霉素来重新编程单核细胞代谢活性,并且寡霉素缺乏针对金黄色葡萄球菌生物膜的抗菌活性。值得注意的是,与全身抗生素相结合,使用寡霉素纳米粒子对单核细胞代谢重编程可有效清除已形成的生物膜。 这些发现表明生物膜相关单核细胞的代谢重编程可能代表 PJI 的一种新治疗方法。
更新日期:2020-03-06
中文翻译:

单核细胞代谢重编程促进促炎活性和金黄色葡萄球菌生物膜清除。
生物膜相关的假体关节感染(PJI)由于不耐受免疫介导的清除和抗生素而导致显着的发病率,其中金黄色葡萄球菌(S. aureus)是最常见的病原体之一。我们之前证明,金黄色葡萄球菌生物膜相关单核细胞被极化为抗炎表型,促炎巨噬细胞的过继转移减轻了生物膜负担,强调了单核细胞/巨噬细胞炎症状态在决定生物膜持久性中的关键作用。白细胞的炎症特性与其代谢状态相关,在这里我们证明生物膜相关单核细胞表现出有利于氧化磷酸化(OxPhos)和较少有氧糖酵解的代谢偏差,以促进其抗炎活性和生物膜持久性。为了改变单核细胞体内代谢并将细胞重新编程至促炎状态,利用纳米颗粒方法将 OxPhos 抑制剂寡霉素递送至单核细胞。使用金黄色葡萄球菌 PJI 小鼠模型,寡霉素纳米颗粒优先被单核细胞内化,代谢组学和 RT-qPCR 分别显示,寡霉素纳米颗粒通过改变代谢和促进浸润单核细胞的促炎特性,显着减少金黄色葡萄球菌生物膜负荷。单独注射寡霉素对单核细胞代谢或生物膜负荷没有影响,这表明需要细胞内递送寡霉素来重新编程单核细胞代谢活性,并且寡霉素缺乏针对金黄色葡萄球菌生物膜的抗菌活性。值得注意的是,与全身抗生素相结合,使用寡霉素纳米粒子对单核细胞代谢重编程可有效清除已形成的生物膜。 这些发现表明生物膜相关单核细胞的代谢重编程可能代表 PJI 的一种新治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号