The Lancet Respiratory Medicine ( IF 38.7 ) Pub Date : 2020-03-05 , DOI: 10.1016/s2213-2600(20)30036-9 Athol U Wells 1 , Kevin R Flaherty 2 , Kevin K Brown 3 , Yoshikazu Inoue 4 , Anand Devaraj 5 , Luca Richeldi 6 , Teng Moua 7 , Bruno Crestani 8 , Wim A Wuyts 9 , Susanne Stowasser 10 , Manuel Quaresma 10 , Rainer-Georg Goeldner 11 , Rozsa Schlenker-Herceg 12 , Martin Kolb 13 ,
|
Background
The INBUILD trial investigated the efficacy and safety of nintedanib versus placebo in patients with progressive fibrosing interstitial lung diseases (ILDs) other than idiopathic pulmonary fibrosis (IPF). We aimed to establish the effects of nintedanib in subgroups based on ILD diagnosis.
Methods
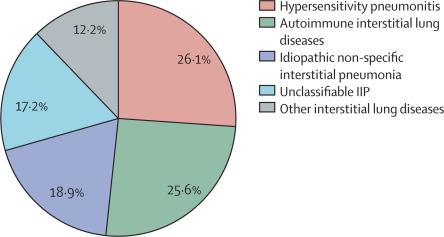
The INBUILD trial was a randomised, double-blind, placebo-controlled, parallel group trial done at 153 sites in 15 countries. Participants had an investigator-diagnosed fibrosing ILD other than IPF, with chest imaging features of fibrosis of more than 10% extent on high resolution CT (HRCT), forced vital capacity (FVC) of 45% or more predicted, and diffusing capacity of the lung for carbon monoxide (DLco) of at least 30% and less than 80% predicted. Participants fulfilled protocol-defined criteria for ILD progression in the 24 months before screening, despite management considered appropriate in clinical practice for the individual ILD. Participants were randomly assigned 1:1 by means of a pseudo-random number generator to receive nintedanib 150 mg twice daily or placebo for at least 52 weeks. Participants, investigators, and other personnel involved in the trial and analysis were masked to treatment assignment until after database lock. In this subgroup analysis, we assessed the rate of decline in FVC (mL/year) over 52 weeks in patients who received at least one dose of nintedanib or placebo in five prespecified subgroups based on the ILD diagnoses documented by the investigators: hypersensitivity pneumonitis, autoimmune ILDs, idiopathic non-specific interstitial pneumonia, unclassifiable idiopathic interstitial pneumonia, and other ILDs. The trial has been completed and is registered with ClinicalTrials.gov, number NCT02999178.
Findings
Participants were recruited between Feb 23, 2017, and April 27, 2018. Of 663 participants who received at least one dose of nintedanib or placebo, 173 (26%) had chronic hypersensitivity pneumonitis, 170 (26%) an autoimmune ILD, 125 (19%) idiopathic non-specific interstitial pneumonia, 114 (17%) unclassifiable idiopathic interstitial pneumonia, and 81 (12%) other ILDs. The effect of nintedanib versus placebo on reducing the rate of FVC decline (mL/year) was consistent across the five subgroups by ILD diagnosis in the overall population (hypersensitivity pneumonitis 73·1 [95% CI −8·6 to 154·8]; autoimmune ILDs 104·0 [21·1 to 186·9]; idiopathic non-specific interstitial pneumonia 141·6 [46·0 to 237·2]; unclassifiable idiopathic interstitial pneumonia 68·3 [−31·4 to 168·1]; and other ILDs 197·1 [77·6 to 316·7]; p=0·41 for treatment by subgroup by time interaction). Adverse events reported in the subgroups were consistent with those reported in the overall population.
Interpretation
The INBUILD trial was not designed or powered to provide evidence for a benefit of nintedanib in specific diagnostic subgroups. However, its results suggest that nintedanib reduces the rate of ILD progression, as measured by FVC decline, in patients who have a chronic fibrosing ILD and progressive phenotype, irrespective of the underlying ILD diagnosis.
Funding
Boehringer Ingelheim.
中文翻译:

在INBUILD试验中,Nintedanib在进行性纤维化性间质性肺病患者中通过间质性肺病诊断进行亚组分析:一项随机,双盲,安慰剂对照,平行组试验。
背景
INBUILD试验研究了Nintedanib与安慰剂在除特发性肺纤维化(IPF)以外的进行性纤维化间质性肺病(ILD)患者中的疗效和安全性。我们旨在根据ILD诊断来确定nintedanib在亚组中的作用。
方法
INBUILD试验是在15个国家/地区的153个地点进行的随机,双盲,安慰剂对照,平行分组试验。参与者经研究者诊断为IPF以外的纤维化ILD,在高分辨率CT(HRCT)上,纤维化的胸部影像学特征超过了10%,预计的肺活量(FVC)为45%或更高,并且弥散能力肺中一氧化碳(DLco)的预测值至少为30%,并低于80%。尽管管理人员认为个体ILD在临床实践中是适当的,但参与者在筛选前的24个月内满足了ILD进展的方案定义标准。通过伪随机数发生器将参与者随机分配为1:1,以每天两次接受150 mg的nintedanib或安慰剂,持续至少52周。参与者,调查员,以及其他参与试验和分析的人员都被掩盖了治疗分配,直到数据库锁定之后。在此亚组分析中,我们根据研究者记录的ILD诊断,评估了五个预先指定的亚组中接受了至少一剂nintedanib或安慰剂的患者在52周内FVC下降的速率(mL /年)。自身免疫性ILD,特发性非特异性间质性肺炎,无法分类的特发性间质性肺炎和其他ILD。该试验已经完成,并已在ClinicalTrials.gov上注册,编号为NCT02999178。我们根据研究者记录的ILD诊断,评估了五个预先指定的亚组中至少接受一剂nintedanib或安慰剂的患者在52周内FVC下降的速率(mL /年):超敏性肺炎,自身免疫性ILD,特发性非特异性间质性肺炎,无法分类的特发性间质性肺炎和其他ILD。该试验已经完成,并已在ClinicalTrials.gov上注册,编号为NCT02999178。我们根据研究者记录的ILD诊断,评估了五个预先指定的亚组中至少接受一剂nintedanib或安慰剂的患者在52周内FVC下降的速率(mL /年):超敏性肺炎,自身免疫性ILD,特发性非特异性间质性肺炎,无法分类的特发性间质性肺炎和其他ILD。该试验已经完成,并已在ClinicalTrials.gov上注册,编号为NCT02999178。
发现
在2017年2月23日至2018年4月27日之间招募了参与者。在663参与者中,至少接受一剂nintedanib或安慰剂的参与者中,有173(26%)人患有慢性超敏性肺炎,170(26%)自身免疫性ILD,125( 19%)特发性非特异性间质性肺炎,114例(17%)无法分类的特发性间质性肺炎和81例(12%)其他ILD。在整个人群中,通过ILD诊断,在五个亚组中,nintedanib与安慰剂对降低FVC下降速率(mL /年)的影响是一致的(超敏性肺炎73·1 [95%CI -8·6至154·8] ;自身免疫性ILD 104·0 [21·1至186·9];特发性非特异性间质性肺炎141·6 [46·0至237·2];无法分类的特发性间质性肺炎68·3 [−31·4至168· 1];其他ILD 197·1 [77·6至316·7];p = 0·41(按时间相互作用按亚组进行治疗)。在亚组中报告的不良事件与在总体人群中报告的不良事件一致。
解释
INBUILD试验的设计或提供的能力并非为特定患者亚组中nintedanib的获益提供证据。但是,其结果表明,无论是潜在的ILD诊断,还是慢性纤维化ILD和进行性表型的患者,nintedanib均可降低FLD下降所测得的ILD进展速度。
资金
勃林格殷格翰。









































 京公网安备 11010802027423号
京公网安备 11010802027423号