Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: a three-arm, open-label, randomised, non-inferiority trial.
The Lancet ( IF 98.4 ) Pub Date : 2020-03-05 , DOI: 10.1016/s0140-6736(19)33222-2 Susan E Krown 1 , Carlee B Moser 2 , Patrick MacPhail 3 , Roy M Matining 2 , Catherine Godfrey 4 , Stephanie R Caruso 5 , Mina C Hosseinipour 6 , Wadzanai Samaneka 7 , Mulinda Nyirenda 8 , Naftali W Busakhala 9 , Fred M Okuku 10 , Josphat Kosgei 11 , Brenda Hoagland 12 , Noluthando Mwelase 3 , Vincent O Oliver 13 , Henriette Burger 14 , Rosie Mngqibisa 15 , Mostafa Nokta 16 , Thomas B Campbell 17 , Margaret Z Borok 18 ,
The Lancet ( IF 98.4 ) Pub Date : 2020-03-05 , DOI: 10.1016/s0140-6736(19)33222-2 Susan E Krown 1 , Carlee B Moser 2 , Patrick MacPhail 3 , Roy M Matining 2 , Catherine Godfrey 4 , Stephanie R Caruso 5 , Mina C Hosseinipour 6 , Wadzanai Samaneka 7 , Mulinda Nyirenda 8 , Naftali W Busakhala 9 , Fred M Okuku 10 , Josphat Kosgei 11 , Brenda Hoagland 12 , Noluthando Mwelase 3 , Vincent O Oliver 13 , Henriette Burger 14 , Rosie Mngqibisa 15 , Mostafa Nokta 16 , Thomas B Campbell 17 , Margaret Z Borok 18 ,
Affiliation
|
BACKGROUND
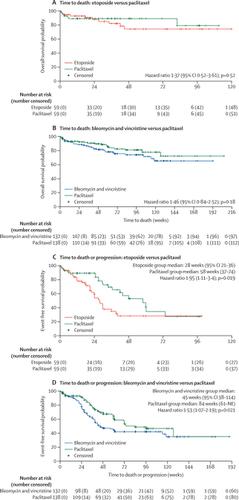
Optimal treatment regimens for AIDS-associated Kaposi sarcoma, a frequent contributor to morbidity and mortality among people with HIV, have not been systematically evaluated in low-income and middle-income countries, where the disease is most common. In this study, we aimed to investigate optimal treatment strategies for advanced stage disease in areas of high prevalence and limited resources.
METHODS
In this open-label, non-inferiority trial, we enrolled people with HIV and advanced stage AIDS-associated Kaposi sarcoma attending 11 AIDS Clinical Trials Group sites in Brazil, Kenya, Malawi, South Africa, Uganda, and Zimbabwe. Eligible participants were randomly assigned (1:1:1) with a centralised computer system to receive either intravenous bleomycin and vincristine or oral etoposide (the investigational arms), or intravenous paclitaxel (the control arm), together with antiretroviral therapy (ART; combined efavirenz, tenofovir disoproxil fumarate, and emtricitabine). The primary outcome was progression-free survival (PFS) at week 48, using a 15% non-inferiority margin to compare the investigational groups against the active control group. Safety was assessed in all eligible treated study participants. The study was registered with ClinicalTrials.gov, NCT01435018.
FINDINGS
334 participants were enrolled between Oct 1, 2013, and March 8, 2018, when the study was closed early due to inferiority of the bleomycin and vincristine plus ART arm, as per the recommendations of the Data and Safety Monitoring Board (DSMB). The etoposide plus ART arm also closed due to inferiority in March, 2016, following a DSMB recommendation. Week-48 PFS rates were higher in the paclitaxel plus ART arm than in both investigational arms. The absolute differences in PFS were -30% (95% CI -52 to -8) for the comparison of paclitaxel plus ART (week 48 PFS 50%, 32 to 67; n=59) and etoposide plus ART (20%, 6 to 33; n=59), and -20% (-33% to -7%) for the comparison of paclitaxel plus ART (64%, 55 to 73; n=138) and bleomycin and vincristine plus ART (44%, 35 to 53; n=132). Both CIs overlapped the non-inferiority margin. The most common adverse events, in 329 eligible participants who began treatment, were neutropenia (48 [15%]), low serum albumin (33 [10%]), weight loss (29 [9%]), and anaemia (28 [9%]), occurring at similar frequency across treatment arms.
INTERPRETATION
Non-inferiority of either investigational intervention was not shown, with paclitaxel plus ART showing superiority to both oral etoposide plus ART and bleomycin and vincristine plus ART, supporting its use in treating advanced AIDS-associated Kaposi sarcoma in resource-limited settings.
FUNDING
US National Institute of Allergy and Infectious Diseases and National Cancer Institute, National Institutes of Health.
中文翻译:

在资源有限的情况下治疗晚期艾滋病相关的卡波济肉瘤:一项三臂,开放标签,随机,非劣效性试验。
背景技术与艾滋病有关的卡波西肉瘤的最佳治疗方案是艾滋病病毒感染者发病和死亡的常见原因,而在这种疾病最常见的低收入和中等收入国家,尚未对其进行系统的评估。在这项研究中,我们旨在研究高流行和资源有限地区晚期疾病的最佳治疗策略。方法在这项开放性,非自卑性试验中,我们招募了参加艾滋病毒和晚期艾滋病相关性卡波西肉瘤的人,这些人参加了在巴西,肯尼亚,马拉维,南非,乌干达和津巴布韦的11个艾滋病临床试验小组。合格的参与者被随机分配(1:1:1)并通过中央计算机系统接受静脉注射博来霉素和长春新碱或口服依托泊苷(研究组),或静脉使用紫杉醇(对照组)以及抗逆转录病毒疗法(ART;联合依非韦伦,替诺福韦富马酸替诺福韦酯和恩曲他滨)。主要结果是第48周的无进展生存期(PFS),使用15%的非自卑裕度将研究组与活动对照组进行比较。在所有合格的接受治疗的研究参与者中评估安全性。该研究已在ClinicalTrials.gov注册,NCT01435018。结果根据数据和安全监测委员会(DSMB)的建议,由于博来霉素和长春新碱加抗逆转录病毒治疗药物的劣势,该研究于2013年10月1日至2018年3月8日之间提前结束,因此有334名受试者入选。依托泊苷加抗逆转录病毒治疗药物的臂膀也因自卑症在DSMB的推荐下于2016年3月关闭。紫杉醇联合抗逆转录病毒治疗组第48周的PFS率高于两个研究组。对于比较紫杉醇加ART(48周PFS 50%,32至67; n = 59)和依托泊苷加ART(20%,6)而言,PFS的绝对差异为-30%(95%CI -52至-8)。至33; n = 59)和紫杉醇加ART(64%,55至73; n = 138)以及博来霉素和长春新碱加ART(44%,-20%(-33%至-7%)) 35至53; n = 132)。两个配置项都覆盖了非劣质性边缘。在329名开始接受治疗的合格参与者中,最常见的不良事件是中性粒细胞减少症(48 [15%]),低血清白蛋白(33 [10%]),体重减轻(29 [9%])和贫血(28 [ 9%]),在各治疗组中的发生频率相似。解释未显示任何一项研究干预的非劣效性,紫杉醇加ART优于口服依托泊苷加ART和博来霉素和长春新碱加ART,在资源有限的情况下支持其治疗晚期艾滋病相关的卡波西肉瘤。资助美国国家过敏和传染病研究所和美国国立卫生研究院国家癌症研究所。
更新日期:2020-04-10
中文翻译:

在资源有限的情况下治疗晚期艾滋病相关的卡波济肉瘤:一项三臂,开放标签,随机,非劣效性试验。
背景技术与艾滋病有关的卡波西肉瘤的最佳治疗方案是艾滋病病毒感染者发病和死亡的常见原因,而在这种疾病最常见的低收入和中等收入国家,尚未对其进行系统的评估。在这项研究中,我们旨在研究高流行和资源有限地区晚期疾病的最佳治疗策略。方法在这项开放性,非自卑性试验中,我们招募了参加艾滋病毒和晚期艾滋病相关性卡波西肉瘤的人,这些人参加了在巴西,肯尼亚,马拉维,南非,乌干达和津巴布韦的11个艾滋病临床试验小组。合格的参与者被随机分配(1:1:1)并通过中央计算机系统接受静脉注射博来霉素和长春新碱或口服依托泊苷(研究组),或静脉使用紫杉醇(对照组)以及抗逆转录病毒疗法(ART;联合依非韦伦,替诺福韦富马酸替诺福韦酯和恩曲他滨)。主要结果是第48周的无进展生存期(PFS),使用15%的非自卑裕度将研究组与活动对照组进行比较。在所有合格的接受治疗的研究参与者中评估安全性。该研究已在ClinicalTrials.gov注册,NCT01435018。结果根据数据和安全监测委员会(DSMB)的建议,由于博来霉素和长春新碱加抗逆转录病毒治疗药物的劣势,该研究于2013年10月1日至2018年3月8日之间提前结束,因此有334名受试者入选。依托泊苷加抗逆转录病毒治疗药物的臂膀也因自卑症在DSMB的推荐下于2016年3月关闭。紫杉醇联合抗逆转录病毒治疗组第48周的PFS率高于两个研究组。对于比较紫杉醇加ART(48周PFS 50%,32至67; n = 59)和依托泊苷加ART(20%,6)而言,PFS的绝对差异为-30%(95%CI -52至-8)。至33; n = 59)和紫杉醇加ART(64%,55至73; n = 138)以及博来霉素和长春新碱加ART(44%,-20%(-33%至-7%)) 35至53; n = 132)。两个配置项都覆盖了非劣质性边缘。在329名开始接受治疗的合格参与者中,最常见的不良事件是中性粒细胞减少症(48 [15%]),低血清白蛋白(33 [10%]),体重减轻(29 [9%])和贫血(28 [ 9%]),在各治疗组中的发生频率相似。解释未显示任何一项研究干预的非劣效性,紫杉醇加ART优于口服依托泊苷加ART和博来霉素和长春新碱加ART,在资源有限的情况下支持其治疗晚期艾滋病相关的卡波西肉瘤。资助美国国家过敏和传染病研究所和美国国立卫生研究院国家癌症研究所。











































 京公网安备 11010802027423号
京公网安备 11010802027423号