Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.micromeso.2020.110145 Mostafa R. Abukhadra , Mohamed Gameel Basyouny , Ahmed M. El-Sherbeeny , Mohammed A. El-Meligy
|
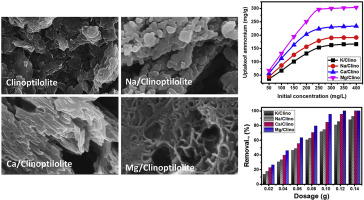
Clinoptilolite natural zeolite was modified by different alkali bearing salts (K, Na, Ca, and Mg) by a green process in the presence of green tea extract as promising green adsorbents for ammonium ions. The modified products displayed significant enhancement in the ion exchange capacities, the surface area, and the morphological properties which was supported by different characterization techniques. The novel modified products achieved stunning adsorption capacities for ammonium ions with experimental qmax of 162 mg/g, 190 mg/g, 230 mg/g and 296 mg/g for K/clinoptilolite, Na/clinoptilolite, Ca/clinoptilolite, and Mg/clinoptilolite, respectively. The theoretical values which were obtained from the equilibrium model are 197.5 mg/g, 219.7 mg/g, 256.2 mg/g, and 340 mg/g for the same products in the order. The adsorption systems of all the prepared adsorbents are highly fitted with both the Pseudo-First order and Pseudo-Second order model with preferences for the first model. Additionally, the uptake of ammonium ions by them is of a monolayer form and of higher fitting with the Langmuir model than the other models considering the correlation coefficient and Chi-squared (χ2) values. The theoretical adsorption energies of K/Clinoptilolite (0.64 KJ/mol), Na/Clinoptilolite (0.76 KJ/mol), Ca/Clinoptilolite (0.9 KJ/mol), and Mg/Clinoptilolite (0.81 KJ/mol) signify physisorption mechanism close to the values of zeolite ion exchange process or coulumbic attractive forces. The modified clinoptilolite products are of high reusability values and applied effectively in the purification of real groundwater and sewage water.
中文翻译:

不同绿色碱改性工艺对斜发沸石表面吸附铵离子的影响;表征与应用
斜发沸石天然沸石在绿茶提取物的存在下通过绿化工艺通过不同的含碱盐(K,Na,Ca和Mg)进行改性,作为有希望的铵离子绿色吸附剂。改性产物在离子交换容量,表面积和形态特性方面显示出显着增强,这得到不同表征技术的支持。新型改性产品在实验q max下达到了惊人的铵离子吸附能力对于K /斜发沸石,Na /斜发沸石,Ca /斜发沸石和Mg /斜发沸石分别为162 mg / g,190 mg / g,230 mg / g和296 mg / g。对于相同产品,从平衡模型获得的理论值依次为197.5 mg / g,219.7 mg / g,256.2 mg / g和340 mg / g。所有制备的吸附剂的吸附系统都高度适合伪一阶模型和伪二阶模型,并且优先选择第一模型。另外,铵离子的通过将它们的摄取是单层形式的并用Langmuir模型不是考虑的相关系数和卡方的其他模型更高嵌合的(χ 2)值。K /斜发沸石(0.64 KJ / mol),Na /斜发沸石(0.76 KJ / mol),Ca /斜发沸石(0.9 KJ / mol)和Mg /斜发沸石(0.81 KJ / mol)的理论吸附能表明其物理吸附机理接近沸石离子交换过程或库仑吸引力的值。改性斜发沸石产品具有较高的可重复使用价值,可有效地用于实际地下水和污水的净化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号