当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An innovative method to enhance manganese and ammonia nitrogen leaching from electrolytic manganese residue by surfactant and anode iron plate
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.hydromet.2020.105311 Jiancheng Shu , Fan Lin , Mengjun Chen , Bing Li , Liang Wei , Jianyi Wang , Zhenggang Luo , Rui Wang
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.hydromet.2020.105311 Jiancheng Shu , Fan Lin , Mengjun Chen , Bing Li , Liang Wei , Jianyi Wang , Zhenggang Luo , Rui Wang
|
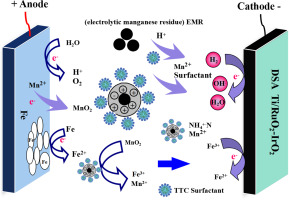
Abstract Electrolytic manganese residue (EMR) contains a lot of manganese (Mn2+) and ammonia nitrogen (NH4+-N), which can cause environmental pollution. In this study, surfactant and anode iron plate were used to synergistically enhance NH4+-N and Mn2+ leaching from EMR. The Characteristics of EMR, Mn2+ and NH4+-N leaching mechanism, and the leaching test were systematically studied. The results showed that the leaching efficiencies of Mn2+ and NH4+-N were 89.4% and 65.9%, respectively, when the current density was 20 mA/cm2 and iron as the anode plate, the mass of H2SO4 was 13 wt%, solid-to-liquid ratio of 1:5 at 20 °C reacting for 120 min. In addition, when the surfactant concentration of tetradecyl trimethylammonium chloride (TTC) was 100 mg/L, the leaching efficiency of Mn2+ and NH4+-N in EMR respectively increased from 89.4% to 97.1% and from 65.9% to 98.4% by adding electric field. The leaching efficiencies of Mn2+ and NH4+-N were 28.5% and 93.7%, respectively, which was higher than that attained under the same condition without an electric field and TTC surfactant. Meanwhile, electric field changed the movement and charge distribution of charged particles on the surface of SiO2 and CaSO4·2H2O particles and released soluble compound salt containing Mn2+ and NH4+-N. TTC surfactant also improved the hydrophilic and hydrophobic properties of the EMR surface and promoted the wetting ability of the particles and the leaching of Mn2+ and NH4+-N. This study shows that TTC surfactant and anode iron plate can enhance NH4+-N and Mn2+leaching from EMR.
中文翻译:

表面活性剂和阳极铁板增强电解锰渣中锰和氨氮浸出的创新方法
摘要 电解锰渣(EMR)中含有大量的锰(Mn2+)和氨氮(NH4+-N),会造成环境污染。在这项研究中,表面活性剂和阳极铁板被用来协同增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。系统研究了EMR、Mn2+和NH4+-N浸出机理的特征以及浸出试验。结果表明,当电流密度为20 mA/cm2、铁为阳极板时,Mn2+和NH4+-N的浸出效率分别为89.4%和65.9%,H2SO4的质量为13 wt%,固对-液比为 1:5 在 20 °C 反应 120 分钟。此外,当十四烷基三甲基氯化铵(TTC)表面活性剂浓度为100 mg/L时,EMR中Mn2+和NH4+-N的浸出效率分别从89.4%提高到97%。1% 和从 65.9% 到 98.4% 通过添加电场。Mn2+和NH4+-N的浸出效率分别为28.5%和93.7%,高于相同条件下无电场和TTC表面活性剂的浸出率。同时,电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。这高于在没有电场和 TTC 表面活性剂的相同条件下获得的结果。同时,电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。这高于在没有电场和 TTC 表面活性剂的相同条件下获得的结果。同时,电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。
更新日期:2020-05-01
中文翻译:

表面活性剂和阳极铁板增强电解锰渣中锰和氨氮浸出的创新方法
摘要 电解锰渣(EMR)中含有大量的锰(Mn2+)和氨氮(NH4+-N),会造成环境污染。在这项研究中,表面活性剂和阳极铁板被用来协同增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。系统研究了EMR、Mn2+和NH4+-N浸出机理的特征以及浸出试验。结果表明,当电流密度为20 mA/cm2、铁为阳极板时,Mn2+和NH4+-N的浸出效率分别为89.4%和65.9%,H2SO4的质量为13 wt%,固对-液比为 1:5 在 20 °C 反应 120 分钟。此外,当十四烷基三甲基氯化铵(TTC)表面活性剂浓度为100 mg/L时,EMR中Mn2+和NH4+-N的浸出效率分别从89.4%提高到97%。1% 和从 65.9% 到 98.4% 通过添加电场。Mn2+和NH4+-N的浸出效率分别为28.5%和93.7%,高于相同条件下无电场和TTC表面活性剂的浸出率。同时,电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。这高于在没有电场和 TTC 表面活性剂的相同条件下获得的结果。同时,电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。这高于在没有电场和 TTC 表面活性剂的相同条件下获得的结果。同时,电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。电场改变了带电粒子在SiO2和CaSO4·2H2O粒子表面的运动和电荷分布,释放出含有Mn2+和NH4+-N的可溶性复合盐。TTC表面活性剂还改善了EMR表面的亲水和疏水性能,促进了颗粒的润湿能力和Mn2+和NH4+-N的浸出。该研究表明,TTC 表面活性剂和阳极铁板可以增强 EMR 中 NH4+-N 和 Mn2+ 的浸出。











































 京公网安备 11010802027423号
京公网安备 11010802027423号