当前位置:
X-MOL 学术
›
BBA Mol. Cell Biol. Lipids
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A role of Gln596 in fine-tuning mammalian ALOX15 specificity, protein stability and allosteric properties.
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 3.9 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.bbalip.2020.158680 Alejandro Cruz 1 , Almerinda Di Venere 2 , Giampiero Mei 2 , Alexander Zhuravlev 3 , Alexey Golovanov 3 , Sabine Stehling 4 , Dagmar Heydeck 4 , José M Lluch 5 , Àngels González-Lafont 5 , Hartmut Kuhn 4 , Igor Ivanov 3
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 3.9 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.bbalip.2020.158680 Alejandro Cruz 1 , Almerinda Di Venere 2 , Giampiero Mei 2 , Alexander Zhuravlev 3 , Alexey Golovanov 3 , Sabine Stehling 4 , Dagmar Heydeck 4 , José M Lluch 5 , Àngels González-Lafont 5 , Hartmut Kuhn 4 , Igor Ivanov 3
Affiliation
|
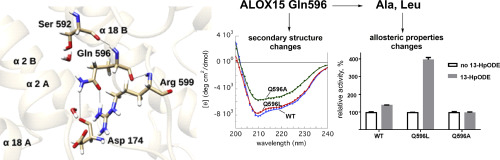
His596 of human ALOX12 has been suggested to interact with the COO--group of arachidonic acid during ALOX catalysis. In mammalian ALOX15 orthologs Gln596 occupies this position and this amino acid exchange might contribute to the functional differences between the two ALOX-isoforms. To explore the role of Gln596 for ALOX15 functionality we mutated this amino acid to different residues in rabbit and human ALOX15 and investigated the impact of these mutations on structural, catalytic and allosteric enzyme properties. To shed light on the molecular basis of the observed functional alterations we performed in silico substrate docking studies and molecular dynamics simulations and also explored the impact of Gln596 exchange on the protein structure. The combined theoretical and experimental data suggest that Gln596 may not directly interact with the COO--group of arachidonic acid. In contrast, mutations at Gln596 destabilize the secondary and tertiary structure of ALOX15 orthologs, which may be related to a disturbance of the electrostatic interaction network with other amino acids in the immediate surrounding. Moreover, our MD-simulations suggest that the geometry of the dimer interface depends on the structure of substrate bound inside the substrate-binding pocket and that Gln596Ala exchange impairs the allosteric properties of the enzyme. Taken together, these data indicate the structural and functional importance of Gln596 for ALOX15 catalysis.
中文翻译:

Gln596在微调哺乳动物ALOX15的特异性,蛋白质稳定性和变构性质中的作用。
已提出人类ALOX12的his596可在ALOX催化过程中与花生四烯酸的COO-基团相互作用。在哺乳动物的ALOX15直系同源物中,Gln596占据了这个位置,并且这种氨基酸交换可能导致了两种ALOX同工型之间的功能差异。为了探索Gln596在ALOX15功能中的作用,我们将该氨基酸突变为兔和人ALOX15中的不同残基,并研究了这些突变对结构,催化和变构酶特性的影响。为了阐明观察到的功能改变的分子基础,我们进行了计算机硅底物对接研究和分子动力学模拟,并探讨了Gln596交换对蛋白质结构的影响。理论和实验的综合数据表明,Gln596可能不与花生四烯酸的COO-基团直接相互作用。相反,Gln596处的突变破坏了ALOX15直系同源物的二级和三级结构的稳定性,这可能与静电相互作用网络与周围其他氨基酸的干扰有关。此外,我们的MD模拟表明,二聚体界面的几何形状取决于结合在底物结合袋内部的底物结构,并且Gln596Ala交换会损害酶的变构性质。总而言之,这些数据表明Gln596对于ALOX15催化的结构和功能重要性。这可能与静电相互作用网络与周围其他氨基酸的干扰有关。此外,我们的MD模拟表明,二聚体界面的几何结构取决于结合在底物结合袋内部的底物结构,并且Gln596Ala交换会损害酶的变构性质。总而言之,这些数据表明Gln596对ALOX15催化的结构和功能重要性。这可能与静电相互作用网络与周围其他氨基酸的干扰有关。此外,我们的MD模拟表明,二聚体界面的几何结构取决于结合在底物结合袋内部的底物结构,并且Gln596Ala交换会损害酶的变构性质。总而言之,这些数据表明Gln596对于ALOX15催化的结构和功能重要性。
更新日期:2020-03-19
中文翻译:

Gln596在微调哺乳动物ALOX15的特异性,蛋白质稳定性和变构性质中的作用。
已提出人类ALOX12的his596可在ALOX催化过程中与花生四烯酸的COO-基团相互作用。在哺乳动物的ALOX15直系同源物中,Gln596占据了这个位置,并且这种氨基酸交换可能导致了两种ALOX同工型之间的功能差异。为了探索Gln596在ALOX15功能中的作用,我们将该氨基酸突变为兔和人ALOX15中的不同残基,并研究了这些突变对结构,催化和变构酶特性的影响。为了阐明观察到的功能改变的分子基础,我们进行了计算机硅底物对接研究和分子动力学模拟,并探讨了Gln596交换对蛋白质结构的影响。理论和实验的综合数据表明,Gln596可能不与花生四烯酸的COO-基团直接相互作用。相反,Gln596处的突变破坏了ALOX15直系同源物的二级和三级结构的稳定性,这可能与静电相互作用网络与周围其他氨基酸的干扰有关。此外,我们的MD模拟表明,二聚体界面的几何形状取决于结合在底物结合袋内部的底物结构,并且Gln596Ala交换会损害酶的变构性质。总而言之,这些数据表明Gln596对于ALOX15催化的结构和功能重要性。这可能与静电相互作用网络与周围其他氨基酸的干扰有关。此外,我们的MD模拟表明,二聚体界面的几何结构取决于结合在底物结合袋内部的底物结构,并且Gln596Ala交换会损害酶的变构性质。总而言之,这些数据表明Gln596对ALOX15催化的结构和功能重要性。这可能与静电相互作用网络与周围其他氨基酸的干扰有关。此外,我们的MD模拟表明,二聚体界面的几何结构取决于结合在底物结合袋内部的底物结构,并且Gln596Ala交换会损害酶的变构性质。总而言之,这些数据表明Gln596对于ALOX15催化的结构和功能重要性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号