当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.jhep.2020.02.027 Stephen A Harrison 1 , Vincent Wai-Sun Wong 2 , Takeshi Okanoue 3 , Natalie Bzowej 4 , Raj Vuppalanchi 5 , Ziad Younes 6 , Anita Kohli 7 , Shiv Sarin 8 , Stephen H Caldwell 9 , Naim Alkhouri 10 , Mitchell L Shiffman 11 , Marianne Camargo 12 , Georgia Li 12 , Kathryn Kersey 12 , Catherine Jia 12 , Yanni Zhu 12 , C Stephen Djedjos 12 , G Mani Subramanian 12 , Robert P Myers 12 , Nadege Gunn 13 , Aasim Sheikh 14 , Quentin M Anstee 15 , Manuel Romero-Gomez 16 , Michael Trauner 17 , Zachary Goodman 18 , Eric J Lawitz 10 , Zobair Younossi 18 , ,
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.jhep.2020.02.027 Stephen A Harrison 1 , Vincent Wai-Sun Wong 2 , Takeshi Okanoue 3 , Natalie Bzowej 4 , Raj Vuppalanchi 5 , Ziad Younes 6 , Anita Kohli 7 , Shiv Sarin 8 , Stephen H Caldwell 9 , Naim Alkhouri 10 , Mitchell L Shiffman 11 , Marianne Camargo 12 , Georgia Li 12 , Kathryn Kersey 12 , Catherine Jia 12 , Yanni Zhu 12 , C Stephen Djedjos 12 , G Mani Subramanian 12 , Robert P Myers 12 , Nadege Gunn 13 , Aasim Sheikh 14 , Quentin M Anstee 15 , Manuel Romero-Gomez 16 , Michael Trauner 17 , Zachary Goodman 18 , Eric J Lawitz 10 , Zobair Younossi 18 , ,
Affiliation
|
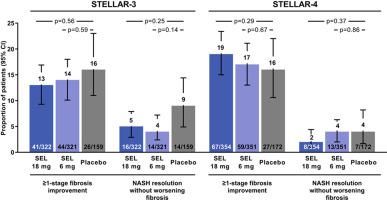
Apoptosis signal-regulating kinase 1 (ASK1) plays a key role in hepatocyte injury, inflammation, and fibrosis in non-alcoholic steatohepatitis (NASH). We evaluated the safety and antifibrotic effect of selonsertib, a selective inhibitor of ASK1, in patients with advanced fibrosis due to NASH. We conducted 2 randomized, double-blind, placebo-controlled, phase III trials of selonsertib in patients with NASH and bridging fibrosis (F3, STELLAR-3) or compensated cirrhosis (F4, STELLAR-4). Patients were randomized 2:2:1 to receive selonsertib 18 mg, selonsertib 6 mg, or placebo once daily for 48 weeks. Liver biopsies were performed at screening and week 48 and non-invasive tests of fibrosis (NITs) were evaluated. The primary efficacy endpoint was the proportion of patients with ≥1-stage improvement in fibrosis without worsening of NASH at week 48. Additional endpoints included changes in NITs, progression to cirrhosis (in STELLAR-3), and liver-related clinical events. Neither trial met the primary efficacy endpoint. In STELLAR-3, fibrosis improvement without worsening of NASH was observed in 10% (31/322, = 0.49 placebo), 12% (39/321, = 0.93 placebo), and 13% (21/159) of patients in the selonsertib 18 mg, selonsertib 6 mg, and placebo groups, respectively. In STELLAR-4, the primary endpoint was achieved in 14% (51/354; = 0.56), 13% (45/351; = 0.93), and 13% (22/172) of patients, respectively. Although selonsertib led to dose-dependent reductions in hepatic phospho-p38 expression indicative of pharmacodynamic activity, it had no significant effect on liver biochemistry, NITs, progression to cirrhosis, or adjudicated clinical events. The rates and types of adverse events were similar among selonsertib and placebo groups. Forty-eight weeks of selonsertib monotherapy had no antifibrotic effect in patients with bridging fibrosis or compensated cirrhosis due to NASH. Patients with non-alcoholic steatohepatitis (NASH) can develop scarring of the liver (fibrosis), including cirrhosis, which increases the risks of liver failure and liver cancer. We tested whether 48 weeks of treatment with selonsertib reduced fibrosis in patients with NASH and advanced liver scarring. We did not find that selonsertib reduced fibrosis in these patients. numbers and .
中文翻译:

Selonsertib 用于治疗 NASH 导致的桥接纤维化或代偿性肝硬化患者:随机 III 期 STELLAR 试验的结果
凋亡信号调节激酶 1 (ASK1) 在非酒精性脂肪性肝炎 (NASH) 的肝细胞损伤、炎症和纤维化中发挥关键作用。我们评估了 Selonsertib(一种 ASK1 选择性抑制剂)在 NASH 引起的晚期纤维化患者中的安全性和抗纤维化作用。我们对 NASH 和桥接纤维化(F3、STELLAR-3)或代偿性肝硬化(F4、STELLAR-4)患者进行了 2 项随机、双盲、安慰剂对照 III 期试验。患者以 2:2:1 的比例随机接受 selonsertib 18 mg、selonsertib 6 mg 或安慰剂,每天一次,持续 48 周。在筛选时和第 48 周进行肝活检,并评估非侵入性纤维化测试 (NIT)。主要疗效终点是第 48 周时纤维化改善≥1 阶段且 NASH 未恶化的患者比例。其他终点包括 NIT 变化、进展为肝硬化(STELLAR-3 中)以及肝脏相关临床事件。两项试验均未达到主要疗效终点。在 STELLAR-3 中,观察到 10%(31/322,= 0.49 安慰剂)、12%(39/321,= 0.93 安慰剂)和 13%(21/159)的患者出现纤维化改善,且 NASH 没有恶化。分别为 selonsertib 18 mg、selonsertib 6 mg 和安慰剂组。在 STELLAR-4 中,主要终点的实现率分别为 14% (51/354; = 0.56)、13% (45/351; = 0.93) 和 13% (22/172) 患者。尽管 selonsertib 导致指示药效活性的肝磷酸化 p38 表达呈剂量依赖性降低,但它对肝脏生化、NIT、肝硬化进展或判定的临床事件没有显着影响。 Selonsertib 组和安慰剂组的不良事件发生率和类型相似。 对于 NASH 导致的桥接纤维化或代偿性肝硬化患者,四十八周的 selonsertib 单一疗法没有抗纤维化作用。非酒精性脂肪性肝炎 (NASH) 患者可能会出现肝脏疤痕(纤维化),包括肝硬化,这会增加肝衰竭和肝癌的风险。我们测试了 48 周的 Selonsertib 治疗是否可以减少 NASH 和晚期肝脏疤痕患者的纤维化。我们没有发现 selonsertib 可以减少这些患者的纤维化。数字和 .
更新日期:2020-03-06
中文翻译:

Selonsertib 用于治疗 NASH 导致的桥接纤维化或代偿性肝硬化患者:随机 III 期 STELLAR 试验的结果
凋亡信号调节激酶 1 (ASK1) 在非酒精性脂肪性肝炎 (NASH) 的肝细胞损伤、炎症和纤维化中发挥关键作用。我们评估了 Selonsertib(一种 ASK1 选择性抑制剂)在 NASH 引起的晚期纤维化患者中的安全性和抗纤维化作用。我们对 NASH 和桥接纤维化(F3、STELLAR-3)或代偿性肝硬化(F4、STELLAR-4)患者进行了 2 项随机、双盲、安慰剂对照 III 期试验。患者以 2:2:1 的比例随机接受 selonsertib 18 mg、selonsertib 6 mg 或安慰剂,每天一次,持续 48 周。在筛选时和第 48 周进行肝活检,并评估非侵入性纤维化测试 (NIT)。主要疗效终点是第 48 周时纤维化改善≥1 阶段且 NASH 未恶化的患者比例。其他终点包括 NIT 变化、进展为肝硬化(STELLAR-3 中)以及肝脏相关临床事件。两项试验均未达到主要疗效终点。在 STELLAR-3 中,观察到 10%(31/322,= 0.49 安慰剂)、12%(39/321,= 0.93 安慰剂)和 13%(21/159)的患者出现纤维化改善,且 NASH 没有恶化。分别为 selonsertib 18 mg、selonsertib 6 mg 和安慰剂组。在 STELLAR-4 中,主要终点的实现率分别为 14% (51/354; = 0.56)、13% (45/351; = 0.93) 和 13% (22/172) 患者。尽管 selonsertib 导致指示药效活性的肝磷酸化 p38 表达呈剂量依赖性降低,但它对肝脏生化、NIT、肝硬化进展或判定的临床事件没有显着影响。 Selonsertib 组和安慰剂组的不良事件发生率和类型相似。 对于 NASH 导致的桥接纤维化或代偿性肝硬化患者,四十八周的 selonsertib 单一疗法没有抗纤维化作用。非酒精性脂肪性肝炎 (NASH) 患者可能会出现肝脏疤痕(纤维化),包括肝硬化,这会增加肝衰竭和肝癌的风险。我们测试了 48 周的 Selonsertib 治疗是否可以减少 NASH 和晚期肝脏疤痕患者的纤维化。我们没有发现 selonsertib 可以减少这些患者的纤维化。数字和 .











































 京公网安备 11010802027423号
京公网安备 11010802027423号