Electrochemistry Communications ( IF 4.7 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.elecom.2020.106705 Satoshi Uchida , Tomohide Katada , Masashi Ishikawa
|
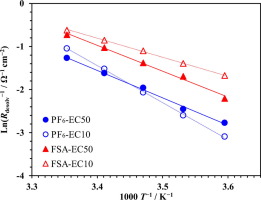
The present paper provides a new perspective regarding the influence of anions on the rate of lithium ion insertion into graphite. We compared the properties of a LiFSA and solvent system with those of LiPF6 system containing the same solvent when the ionic conductivity of these systems is comparable, to elucidate that better rate properties of the former system than the latter is well reflected in the lower desolvation resistance (Rdesolv) and its lower activation energy (Eadesolv) of the former. The solvation circumstances around Li+ are similar for both the LiFSA and LiPF6 systems, while a significant interaction between Li+ and an anion is observed only in the former. In addition to the previously proposed hypothesis that the Eadesolv reflects the energy required to desolvate Li+ from the last solvating molecule, the present result points to the necessity of considering the influence of the counter anion.
中文翻译:

碳酸盐基电解质中锂离子配位对锂离子嵌入石墨电极的动力学的影响
本文提供了有关阴离子对锂离子插入石墨速率的影响的新观点。当这些系统的离子电导率相当时,我们将LiFSA和溶剂系统的性能与包含相同溶剂的LiPF 6系统的性能进行了比较,以阐明前者系统比后者具有更好的速率特性可以很好地反映在较低的去溶剂化中电阻(R desolv)及其较低的活化能(E a desolv)。LiFSA和LiPF 6系统在Li +周围的溶剂化情况相似,而Li +之间的显着相互作用仅在前者中观察到阴离子。除了先前提出的E a解溶剂反应了从最后一个溶剂化分子上解离Li +所需的能量的假设外,本研究结果还表明有必要考虑抗衡阴离子的影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号