当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of orally active indirubin-3'-oxime derivatives as potent type 1 FLT3 inhibitors for acute myeloid leukemia.
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.ejmech.2020.112205 Pyeonghwa Jeong 1 , Yeongyu Moon 2 , Je-Heon Lee 3 , So-Deok Lee 3 , Jiyeon Park 3 , Jungeun Lee 3 , Jiheon Kim 3 , Hyo Jeong Lee 4 , Na Yoon Kim 5 , Jungil Choi 2 , Jeong Doo Heo 2 , Ji Eun Shin 6 , Hyun Woo Park 6 , Yoon-Gyoon Kim 5 , Sun-Young Han 4 , Yong-Chul Kim 7
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.ejmech.2020.112205 Pyeonghwa Jeong 1 , Yeongyu Moon 2 , Je-Heon Lee 3 , So-Deok Lee 3 , Jiyeon Park 3 , Jungeun Lee 3 , Jiheon Kim 3 , Hyo Jeong Lee 4 , Na Yoon Kim 5 , Jungil Choi 2 , Jeong Doo Heo 2 , Ji Eun Shin 6 , Hyun Woo Park 6 , Yoon-Gyoon Kim 5 , Sun-Young Han 4 , Yong-Chul Kim 7
Affiliation
|
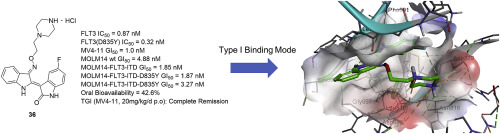
FMS-like receptor tyrosine kinase-3 (FLT3) is expressed on acute leukemia cells and is implicated in the survival, proliferation and differentiation of hematopoietic cells in most acute myeloid leukemia (AML) patients. Despite recent achievements in the development of FLT3-targeted small-molecule drugs, there are still unmet medical needs related to kinase selectivity and the progression of some mutant forms of FLT3. Herein, we describe the discovery of novel orally available type 1 FLT3 inhibitors from structure-activity relationship (SAR) studies for the optimization of indirubin derivatives with biological and pharmacokinetic profiles as potential therapeutic agents for AML. The SAR exploration provided important structural insights into the key substituents for potent inhibitory activities of FLT3 and in MV4-11 cells. The profile of the most optimized inhibitor (36) showed IC50 values of 0.87 and 0.32 nM against FLT3 and FLT3/D835Y, respectively, along with potent inhibition against MV4-11 and FLT3/D835Y expressed MOLM14 cells with a GI50 value of 1.0 and 1.87 nM, respectively. With the high oral bioavailability of 42.6%, compound 36 displayed significant in vivo antitumor activity by oral administration of 20 mg/kg once daily dosing schedule for 21 days in a mouse xenograft model. The molecular docking study of 36 in the homology model of the DFG-in conformation of FLT3 resulted in a reasonable binding mode in type 1 kinases similar to the reported type 1 FLT3 inhibitors Crenolanib and Gilteritinib.
中文翻译:

口服活性靛玉红3'-肟衍生物作为急性髓性白血病有效的1型FLT3抑制剂的发现。
FMS样受体酪氨酸激酶3(FLT3)在急性白血病细胞上表达,并与大多数急性髓细胞白血病(AML)患者的造血细胞存活,增殖和分化有关。尽管在靶向FLT3的小分子药物的开发方面取得了新的成就,但仍存在与激酶选择性和某些FLT3突变形式的进展有关的医疗需求尚未得到满足。在这里,我们描述了从结构-活性关系(SAR)研究中发现的新型口服可用的1型FLT3抑制剂,以研究具有生物学和药代动力学特性的靛玉红衍生物作为AML的潜在治疗剂。SAR探索为FLT3和MV4-11细胞有效抑制活性的关键取代基提供了重要的结构见解。最优化的抑制剂(36)的图谱显示,其对FLT3和FLT3 / D835Y的IC50值分别为0.87和0.32 nM,同时对MV4-11和FLT3 / D835Y表达的MOLM14细胞具有有效的抑制作用,GI50值为1.0和1.87分别为nM。在42.6%的高口服生物利用度下,化合物36通过在小鼠异种移植模型中每天口服一次20 mg / kg的给药方案持续21天,显示出显着的体内抗肿瘤活性。在FLT3的DFG-in构象同源模型中对36进行的分子对接研究导致与报道的1型FLT3抑制剂Crenolanib和Gilteritinib相似的1型激酶的合理结合模式。以及有效抑制MV4-11和FLT3 / D835Y表达的GI50值分别为1.0和1.87 nM的MOLM14细胞。在42.6%的高口服生物利用度下,化合物36通过在小鼠异种移植模型中每天口服一次20 mg / kg的给药方案持续21天,显示出显着的体内抗肿瘤活性。在FLT3的DFG-in构象同源模型中对36进行的分子对接研究导致与报道的1型FLT3抑制剂Crenolanib和Gilteritinib相似的1型激酶的合理结合模式。以及有效抑制MV4-11和FLT3 / D835Y表达的GI50值分别为1.0和1.87 nM的MOLM14细胞。在42.6%的高口服生物利用度下,化合物36通过在小鼠异种移植模型中每天口服一次20 mg / kg的给药方案持续21天,显示出显着的体内抗肿瘤活性。在FLT3的DFG-in构象同源模型中对36进行的分子对接研究导致与报道的1型FLT3抑制剂Crenolanib和Gilteritinib相似的1型激酶的合理结合模式。
更新日期:2020-03-06
中文翻译:

口服活性靛玉红3'-肟衍生物作为急性髓性白血病有效的1型FLT3抑制剂的发现。
FMS样受体酪氨酸激酶3(FLT3)在急性白血病细胞上表达,并与大多数急性髓细胞白血病(AML)患者的造血细胞存活,增殖和分化有关。尽管在靶向FLT3的小分子药物的开发方面取得了新的成就,但仍存在与激酶选择性和某些FLT3突变形式的进展有关的医疗需求尚未得到满足。在这里,我们描述了从结构-活性关系(SAR)研究中发现的新型口服可用的1型FLT3抑制剂,以研究具有生物学和药代动力学特性的靛玉红衍生物作为AML的潜在治疗剂。SAR探索为FLT3和MV4-11细胞有效抑制活性的关键取代基提供了重要的结构见解。最优化的抑制剂(36)的图谱显示,其对FLT3和FLT3 / D835Y的IC50值分别为0.87和0.32 nM,同时对MV4-11和FLT3 / D835Y表达的MOLM14细胞具有有效的抑制作用,GI50值为1.0和1.87分别为nM。在42.6%的高口服生物利用度下,化合物36通过在小鼠异种移植模型中每天口服一次20 mg / kg的给药方案持续21天,显示出显着的体内抗肿瘤活性。在FLT3的DFG-in构象同源模型中对36进行的分子对接研究导致与报道的1型FLT3抑制剂Crenolanib和Gilteritinib相似的1型激酶的合理结合模式。以及有效抑制MV4-11和FLT3 / D835Y表达的GI50值分别为1.0和1.87 nM的MOLM14细胞。在42.6%的高口服生物利用度下,化合物36通过在小鼠异种移植模型中每天口服一次20 mg / kg的给药方案持续21天,显示出显着的体内抗肿瘤活性。在FLT3的DFG-in构象同源模型中对36进行的分子对接研究导致与报道的1型FLT3抑制剂Crenolanib和Gilteritinib相似的1型激酶的合理结合模式。以及有效抑制MV4-11和FLT3 / D835Y表达的GI50值分别为1.0和1.87 nM的MOLM14细胞。在42.6%的高口服生物利用度下,化合物36通过在小鼠异种移植模型中每天口服一次20 mg / kg的给药方案持续21天,显示出显着的体内抗肿瘤活性。在FLT3的DFG-in构象同源模型中对36进行的分子对接研究导致与报道的1型FLT3抑制剂Crenolanib和Gilteritinib相似的1型激酶的合理结合模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号