当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
1,3-Dipolar cycloaddition of N-allyl substituted polycyclic derivatives of isoindole-1,3-dione with nitrones and nitrile oxides: An experimental and theoretical investigation
Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.tet.2020.131104 Mariia M. Efremova , Alexander P. Molchanov , Alexander S. Novikov , Galina L. Starova , Anna A. Muryleva , Alexander V. Slita , Vladimir V. Zarubaev
中文翻译:

N-烯丙基取代的异吲哚-1,3-二酮的多环衍生物与硝酮和腈的1,3-偶极环加成反应:实验和理论研究
更新日期:2020-03-06
Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.tet.2020.131104 Mariia M. Efremova , Alexander P. Molchanov , Alexander S. Novikov , Galina L. Starova , Anna A. Muryleva , Alexander V. Slita , Vladimir V. Zarubaev
|
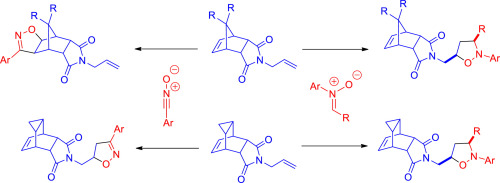
Cycloaddition reactions of N-allyl substituted polycyclic derivatives of isoindole-1,3-dione with nitrones proceeds regio- and stereoselectively on the double bond of the N-allyl substituent giving substituted isoxazolidines with good yields. Regioselectivity of the cycloaddition of this dipolarophiles with nitrile oxides depends on the structure of unsaturated hydrocarbon scaffold and the reaction selectively leads to adducts on endocyclic or the double bond of the N-allyl substituent. DFT calculations were used to investigate the reasons of the selectivity.
中文翻译:

N-烯丙基取代的异吲哚-1,3-二酮的多环衍生物与硝酮和腈的1,3-偶极环加成反应:实验和理论研究
的环加成反应ñ -烯丙基取代的异吲哚-1,3-二酮的合多环衍生物与硝酮收益上的双键区域选择性和立体选择性地Ñ具有良好产率-烯丙基取代基取代给人异恶唑烷。该双亲亲物与腈氧化物的环加成反应的区域选择性取决于不饱和烃骨架的结构,该反应选择性地导致N-烯丙基取代基的内环或双键上的加合物。DFT计算用于研究选择性的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号