Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.tetlet.2020.151805 Imen Boualia , Abdelmadjid Debache , Raouf Boulcina , Thierry Roisnel , Fabienne Berrée , Joëlle Vidal , Bertrand Carboni
|
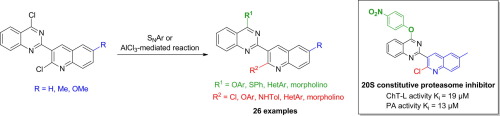
A new series of 3-(quinazol-2-yl)-quinolines was synthesized by SNAr reaction from easily prepared 4-chloro-2-(2-chloroquinolin-3-yl)quinazolines and a range of phenols and thiophenol as nucleophiles. The AlCl3-mediated C–C bond formation was also successfully exploited to introduce aryl and hereroaryl substituents on one or both heterocyclic units. These procedures afford efficient syntheses of polysubstituted 3-(quinazol-2-yl)-quinolines in few steps and high yields. Some of these polysubstituted 3-(quinazol-2-yl)-quinolines inhibit the human 20S proteasome.
中文翻译:

S N Ar和氯化铝诱导的(杂)芳基化反应合成新型3-(喹唑-2-基)喹啉和作为蛋白酶体抑制剂的生物学评价
通过S N Ar反应,由易于制备的4-氯-2-(2-氯喹啉-3-基)喹唑啉和一系列酚和苯硫酚作为亲核试剂,合成了一系列新的3-(喹唑-2-基)-喹啉。AlCl 3介导的C–C键的形成也已成功地用于在一个或两个杂环单元上引入芳基和异芳基取代基。这些方法可以在几个步骤中以高收率有效地合成多取代的3-(喹唑-2-基)喹啉。这些多取代的3-(喹唑-2-基)-喹啉中的一些抑制人20S蛋白酶体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号