Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.tetlet.2020.151806 Fei-Yu Chen , Li Xiang , Gu Zhan , Hong Liu , Bin Kang , Shu-Cang Zhang , Cheng Peng , Bo Han
|
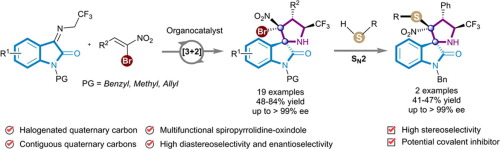
The highly stereoselective synthesis of pyrrolidine-fused spirooxindole derivatives bearing a carbon-halogen bond and contiguous quaternary carbon stereocenters was achieved via a [3+2] cycloaddition reaction. This method provided facile access to a collection of enantiomerically pure spiro[pyrrolidin-3,2’-oxindoles] containing halogenated contiguous quaternary carbon stereocenters in good to high yields (48-84%) and excellent stereoselectivity (up to >20:1 dr and >99% ee). The halogen-containing products can be stereoselectively transformed into sulfurated derivatives via nucleophilic substitution (SN2) reactions, indicating that they may serve as candidates in the development of covalent inhibitors with potential biological activity.
中文翻译:

含卤化连续季碳立体中心的吡咯烷基螺螺辛酯的高度立体选择性有机催化合成
通过[3 + 2]环加成反应实现了具有碳-卤素键和连续的季碳立体中心的吡咯烷稠合的螺并氧杂吲哚衍生物的高度立体选择性合成。该方法可轻松获得对映体纯的螺[吡咯烷-3,2'-羟吲哚]的集合,其中包含卤化连续的季碳立体中心,具有良好至高产率(48-84%)和出色的立体选择性(高达> 20:1 dr和> 99%ee)。可以通过亲核取代(S N 2)反应将含卤素的产物立体选择性地转化为硫化衍生物,这表明它们可以作为具有潜在生物活性的共价抑制剂的开发候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号