Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-03-06 , DOI: 10.1016/j.apcatb.2020.118859 Shuyue Chen , Jeremie Zaffran , Bo Yang
|
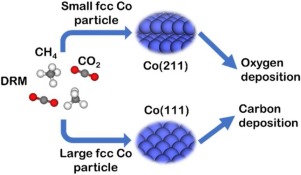
Cobalt shows high catalytic activity for the important dry reforming of methane (DRM) reaction. However, it is prone to deactivation and the corresponding mechanism remains controversial. In this work, we combined density functional theory calculations and microkinetic modeling to study the active site and reaction mechanism of Co catalyzed DRM reaction, employing face centered cubic Co(111) and Co(211) as models. It was found that the step site over Co(211) is the active site for the reaction, and on Co(111), the C + O and CH + O paths are the preferred reaction pathways, while the C + O path is dominant on Co(211). The dissociation of CH4 is the rate-controlling step of DRM over both Co(111) and Co(211). We found that Co(111) is mainly deactivated due to carbon deposition whilst Co(211) undergoes surface oxidization. In addition, Co(111) tends to follow the surface carbon coupling mechanism, and surface carbon clusters formed will lead to catalyst deactivation.
中文翻译:

钴催化剂上甲烷的干重整:催化剂失活的反应动力学和机理的理论见解
对于重要的甲烷干重整(DRM)反应,钴显示出高催化活性。然而,它容易失活,并且相应的机制仍存在争议。在这项工作中,我们以面心立方Co(111)和Co(211)为模型,结合了密度泛函理论计算和微观动力学模型,研究了Co催化DRM反应的活性位点和反应机理。发现Co(211)上的阶梯位点是反应的活性位点,在Co(111)上,C + O和CH + O路径是首选的反应路径,而C + O路径占主导在Co(211)上。CH 4的解离是在Co(111)和Co(211)上DRM的速率控制步骤。我们发现,Co(111)主要由于碳沉积而失活,而Co(211)则经历表面氧化。此外,Co(111)倾向于遵循表面碳偶合机理,并且形成的表面碳簇会导致催化剂失活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号