当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Incorporation of the Nonproteinogenic Amino Acid β-Methylamino-alanine Affects Amyloid β Fibril Properties and Toxicity.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-03-17 , DOI: 10.1021/acschemneuro.9b00660 Alexander Korn 1 , Corinna Höfling 2 , Ulrike Zeitschel 2 , Martin Krueger 3 , Steffen Roßner 2 , Daniel Huster 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-03-17 , DOI: 10.1021/acschemneuro.9b00660 Alexander Korn 1 , Corinna Höfling 2 , Ulrike Zeitschel 2 , Martin Krueger 3 , Steffen Roßner 2 , Daniel Huster 1
Affiliation
|
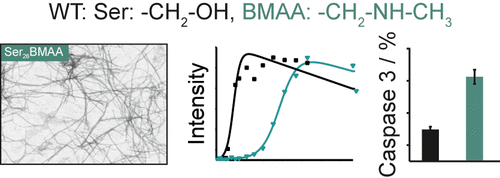
The nonproteinogenic amino acid β-methylamino alarelevant example for environmental hazards are nonnine (BMAA) is a neurotoxin and represents a potential risk factor for neurodegenerative diseases. Despite intense research over the last years, the pathological mechanism of BMAA is still unclear. One of the main open questions is whether BMAA can be misincorporated into proteins, especially as a substitute for serine, and whether this has structural and functional consequences for the afflicted proteins leading to early onset neurodegeneration. In this study, we hypothesize that BMAA was indeed incorporated into Aβ40 molecules and study the structural and dynamical consequences of such misincorporation along with the effect such mutated Aβ40 peptides have on neuronal cells. We used the synthetic β-amyloid peptide (Aβ40), a known key player in the development of Alzheimer’s disease, to incorporate BMAA substitutions at three different positions in the peptide sequence: Ser8BMAA at the peptide’s N-terminus, Phe19BMAA in the hydrophobic core region, and S26BMAA in the flexible turn region of Aβ40 fibrils. We performed a set of biophysical experiments including fluorescence, circular dichroism, solid-state NMR spectroscopy, transmission electron microscopy, and X-ray diffraction to investigate structural and functional aspects of the mutated peptides compared to wildtype Aβ40. All variants showed high structural tolerance to BMAA misincorporation. In contrast, the cellular response and neuronal survival were affected in a mutation site-specific manner. As a consequence, we can state from the physicochemical point of view that, if BMAA was misincorporated into proteins, it could indeed represent a risk factor that could potentially play a role in neurodegeneration. Further research addressing the role of BMAA, especially its protein-associated form, should be performed to obtain a better understanding of neurodegenerative diseases and to develop new therapeutic strategies.
中文翻译:

非蛋白原性氨基酸β-甲基氨基丙氨酸的掺入会影响β-淀粉样蛋白的原纤维特性和毒性。
造成环境危害的非蛋白质氨基酸β-甲基氨基芳香族化合物的例子是壬酸(BMAA)是一种神经毒素,代表神经退行性疾病的潜在危险因素。尽管最近几年进行了大量研究,但BMAA的病理机制仍不清楚。主要的开放性问题之一是BMAA是否可以错误地掺入蛋白质中,特别是作为丝氨酸的替代物,以及是否对患病的蛋白质造成结构和功能性后果,从而导致早期神经变性。在这项研究中,我们假设BMAA确实掺入了Aβ40分子中,并研究了这种错误掺入的结构和动力学后果以及这种突变的Aβ40肽对神经元细胞有作用。我们使用了合成的β-淀粉样蛋白肽(Aβ40),它是阿尔茨海默氏病发展过程中的关键角色,在肽序列的三个不同位置引入了BMAA取代:在肽N端的Ser 8 BMAA,Phe 19 BMAA疏水核心区域中的S 26 BMAA和Aβ40原纤维的柔性转向区域中的S 26 BMAA 。我们进行了一系列生物物理实验,包括荧光,圆二色性,固态NMR光谱,透射电子显微镜和X射线衍射,以研究与野生型Aβ40相比突变肽的结构和功能方面。所有变体均显示出对BMAA错掺的高结构耐受性。相反,细胞反应和神经元存活以突变位点特异性方式受到影响。结果,我们可以从理化的角度指出,如果BMAA被错误地掺入蛋白质中,它实际上可能代表了可能在神经退行性疾病中起作用的危险因素。应进行针对BMAA的作用的进一步研究,尤其是其蛋白质相关形式的作用,以更好地了解神经退行性疾病并制定新的治疗策略。
更新日期:2020-03-19
中文翻译:

非蛋白原性氨基酸β-甲基氨基丙氨酸的掺入会影响β-淀粉样蛋白的原纤维特性和毒性。
造成环境危害的非蛋白质氨基酸β-甲基氨基芳香族化合物的例子是壬酸(BMAA)是一种神经毒素,代表神经退行性疾病的潜在危险因素。尽管最近几年进行了大量研究,但BMAA的病理机制仍不清楚。主要的开放性问题之一是BMAA是否可以错误地掺入蛋白质中,特别是作为丝氨酸的替代物,以及是否对患病的蛋白质造成结构和功能性后果,从而导致早期神经变性。在这项研究中,我们假设BMAA确实掺入了Aβ40分子中,并研究了这种错误掺入的结构和动力学后果以及这种突变的Aβ40肽对神经元细胞有作用。我们使用了合成的β-淀粉样蛋白肽(Aβ40),它是阿尔茨海默氏病发展过程中的关键角色,在肽序列的三个不同位置引入了BMAA取代:在肽N端的Ser 8 BMAA,Phe 19 BMAA疏水核心区域中的S 26 BMAA和Aβ40原纤维的柔性转向区域中的S 26 BMAA 。我们进行了一系列生物物理实验,包括荧光,圆二色性,固态NMR光谱,透射电子显微镜和X射线衍射,以研究与野生型Aβ40相比突变肽的结构和功能方面。所有变体均显示出对BMAA错掺的高结构耐受性。相反,细胞反应和神经元存活以突变位点特异性方式受到影响。结果,我们可以从理化的角度指出,如果BMAA被错误地掺入蛋白质中,它实际上可能代表了可能在神经退行性疾病中起作用的危险因素。应进行针对BMAA的作用的进一步研究,尤其是其蛋白质相关形式的作用,以更好地了解神经退行性疾病并制定新的治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号