当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Specific 89Zr- and 111In-Radiolabeling and In Vivo Evaluation of Glycan-free Antibodies by Azide-Alkyne Cycloaddition with a Non-natural Amino Acid.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-03-05 , DOI: 10.1021/acs.bioconjchem.0c00100 Shin Hye Ahn 1 , Brett A Vaughn 1 , Willy A Solis 2 , Mark L Lupher 2 , Trevor J Hallam 2 , Eszter Boros 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-03-05 , DOI: 10.1021/acs.bioconjchem.0c00100 Shin Hye Ahn 1 , Brett A Vaughn 1 , Willy A Solis 2 , Mark L Lupher 2 , Trevor J Hallam 2 , Eszter Boros 1
Affiliation
|
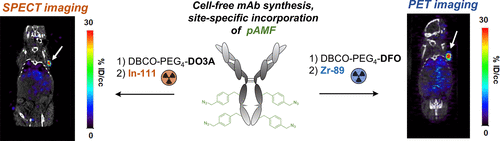
Antibody–drug conjugates (ADCs) are a class of targeted therapeutics consisting of a monoclonal antibody coupled to a cytotoxic payload. Various bioconjugation methods for producing site-specific ADCs have been reported recently, in efforts to improve immunoreactivity and pharmacokinetics and minimize batch variance—potential issues associated with first-generation ADCs prepared via stochastic peptide coupling of lysines or reduced cysteines. Recently, cell-free protein synthesis of antibodies incorporating para-azidomethyl phenylalanine (pAMF) at specific locations within the protein sequence has emerged as a means to generate antibody–drug conjugates with strictly defined drug–antibody-ratio, leading to ADCs with markedly improved stability, activity, and specificity. The incorporation of pAMF enables the conjugation of payloads functionalized for strain-promoted azide–alkyne cycloaddition. Here, we introduce two dibenzylcyclooctyne-functionalized bifunctional chelators that enable the incorporation of radioisotopes for positron emission tomography with 89Zr (t1/2 = 78.4 h, β+ = 395 keV (22%), γ = 897 keV) or single photon emission computed tomography with 111In (t1/2 = 67.3 h, γ = 171 keV (91%), 245 keV (94%)) under physiologically compatible conditions. We show that the corresponding radiolabeled conjugates with site-specifically functionalized antibodies targeting HER2 are amenable to targeted molecular imaging of HER2+ expressing tumor xenografts in mice and exhibit a favorable biodistribution profile in comparison with conventional, glycosylated antibody conjugates generated by stochastic bioconjugation.
中文翻译:

通过叠氮基-炔烃环加成与非天然氨基酸对无糖聚糖抗体进行定点89Zr和111In放射性标记和体内评估。
抗体-药物偶联物(ADC)是一类靶向治疗药物,由与细胞毒性有效载荷偶联的单克隆抗体组成。近年来,已报道了各种用于产生位点特异性ADC的生物共轭方法,以努力改善免疫反应性和药代动力学,并最大程度地减少批次差异,这是与通过赖氨酸或还原半胱氨酸的随机肽偶联制备的第一代ADC相关的潜在问题。近日,无细胞结合的抗体的蛋白质合成第在蛋白质序列内特定位置的-叠氮基甲基苯丙氨酸(pAMF)已经成为一种产生具有严格定义的药物-抗体比率的抗体-药物偶联物的方法,从而导致ADC的稳定性,活性和特异性显着提高。pAMF的掺入可以缀合为应变促进的叠氮化物-炔烃环加成而功能化的有效负载。在这里,我们介绍了两种二苄基环辛炔官能化的双功能螯合剂,这些螯合剂能够以89 Zr(t 1/2 = 78.4 h,β + = 395 keV(22%),γ= 897 keV)或单光子掺入用于正电子发射断层扫描的放射性同位素发射计算机断层扫描(111 In(t 1/2在生理上相容的条件下= 67.3 h,γ= 171 keV(91%),245 keV(94%)。我们显示,具有针对HER2的位点特异性功能化抗体的相应放射性标记的缀合物适合于小鼠中表达HER2 +的肿瘤异种移植物的靶向分子成像,并且与通过随机生物缀合产生的常规糖基化抗体缀合物相比表现出良好的生物分布特征。
更新日期:2020-04-23
中文翻译:

通过叠氮基-炔烃环加成与非天然氨基酸对无糖聚糖抗体进行定点89Zr和111In放射性标记和体内评估。
抗体-药物偶联物(ADC)是一类靶向治疗药物,由与细胞毒性有效载荷偶联的单克隆抗体组成。近年来,已报道了各种用于产生位点特异性ADC的生物共轭方法,以努力改善免疫反应性和药代动力学,并最大程度地减少批次差异,这是与通过赖氨酸或还原半胱氨酸的随机肽偶联制备的第一代ADC相关的潜在问题。近日,无细胞结合的抗体的蛋白质合成第在蛋白质序列内特定位置的-叠氮基甲基苯丙氨酸(pAMF)已经成为一种产生具有严格定义的药物-抗体比率的抗体-药物偶联物的方法,从而导致ADC的稳定性,活性和特异性显着提高。pAMF的掺入可以缀合为应变促进的叠氮化物-炔烃环加成而功能化的有效负载。在这里,我们介绍了两种二苄基环辛炔官能化的双功能螯合剂,这些螯合剂能够以89 Zr(t 1/2 = 78.4 h,β + = 395 keV(22%),γ= 897 keV)或单光子掺入用于正电子发射断层扫描的放射性同位素发射计算机断层扫描(111 In(t 1/2在生理上相容的条件下= 67.3 h,γ= 171 keV(91%),245 keV(94%)。我们显示,具有针对HER2的位点特异性功能化抗体的相应放射性标记的缀合物适合于小鼠中表达HER2 +的肿瘤异种移植物的靶向分子成像,并且与通过随机生物缀合产生的常规糖基化抗体缀合物相比表现出良好的生物分布特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号