当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Investigation of the Nickel-Catalyzed Carbonylation of Alcohols
Organometallics ( IF 2.5 ) Pub Date : 2020-03-06 , DOI: 10.1021/acs.organomet.0c00082 Sara Sabater 1 , Maximilian Menche 1, 2 , Tamal Ghosh 1 , Saskia Krieg 3 , Katharina S. L. Rück 4 , Rocco Paciello 4 , Ansgar Schäfer 2 , Peter Comba 3 , A. Stephen K. Hashmi 1, 5 , Thomas Schaub 1, 4
Organometallics ( IF 2.5 ) Pub Date : 2020-03-06 , DOI: 10.1021/acs.organomet.0c00082 Sara Sabater 1 , Maximilian Menche 1, 2 , Tamal Ghosh 1 , Saskia Krieg 3 , Katharina S. L. Rück 4 , Rocco Paciello 4 , Ansgar Schäfer 2 , Peter Comba 3 , A. Stephen K. Hashmi 1, 5 , Thomas Schaub 1, 4
Affiliation
|
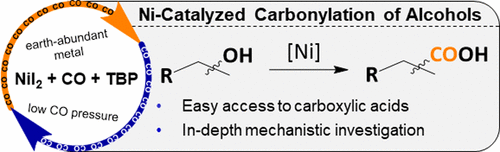
The carbonylation of alcohols represents a straightforward and atom-efficient methodology for the preparation of carboxylic acids. It is desirable to perform these reactions under precious metal-free and low-pressure conditions, with regioselectivity control. In this work, we present a detailed mechanistic study of a catalytic system based on NiI2, which can carbonylate benzylic alcohols in a highly regioselective manner to the corresponding branched carboxylic acids, core motifs for nonsteroidal drugs. The combination of catalytic amounts of nickel and iodide is crucial for efficient catalytic and regioselective conversion. Quantum-chemical computations were used to evaluate the underlying mechanistic processes. They revealed that a combination of two mechanisms is responsible for the observed reactivity and that the oxidative addition of alkyl halides to the Ni(0) species follows a radical oxidation pathway via two one-electron steps.
中文翻译:

镍催化醇羰基化的机理研究
醇的羰基化代表了一种简单且原子高效的制备羧酸的方法。期望在区域选择性控制下在无贵金属和低压条件下进行这些反应。在这项工作中,我们提出了基于NiI 2的催化系统的详细机理研究。可以以高度区域选择性的方式将苄醇羰基化为相应的支链羧酸,这是非甾体药物的核心图案。催化量的镍和碘化物的组合对于有效的催化和区域选择性转化至关重要。量子化学计算被用来评估潜在的机械过程。他们发现,两种机制的结合是所观察到的反应性的原因,烷基卤化物向Ni(0)物种的氧化加成遵循通过两个单电子步骤的自由基氧化途径。
更新日期:2020-03-06
中文翻译:

镍催化醇羰基化的机理研究
醇的羰基化代表了一种简单且原子高效的制备羧酸的方法。期望在区域选择性控制下在无贵金属和低压条件下进行这些反应。在这项工作中,我们提出了基于NiI 2的催化系统的详细机理研究。可以以高度区域选择性的方式将苄醇羰基化为相应的支链羧酸,这是非甾体药物的核心图案。催化量的镍和碘化物的组合对于有效的催化和区域选择性转化至关重要。量子化学计算被用来评估潜在的机械过程。他们发现,两种机制的结合是所观察到的反应性的原因,烷基卤化物向Ni(0)物种的氧化加成遵循通过两个单电子步骤的自由基氧化途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号