当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-03-06 , DOI: 10.1021/acs.accounts.9b00635 Xiang-Yu Chen 1, 2 , Zhong-Hua Gao 1 , Song Ye 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-03-06 , DOI: 10.1021/acs.accounts.9b00635 Xiang-Yu Chen 1, 2 , Zhong-Hua Gao 1 , Song Ye 1, 2
Affiliation
|
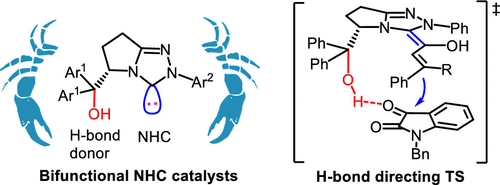
In nature, enzymes are a powerful medium for the construction of enantiomerically pure chemicals, which always inspires synthetic chemists to explore new catalysts to imitate the enzyme machinery for asymmetric transformations. Vitamin B1, a bifunctional thiazolium N-heterocyclic carbene (NHC) precursor, is the coenzyme for transketolase. In the past two decades, a series of chiral NHCs, including monocyclic, bicyclic, tetracyclic, and even bridged ones, have been synthesized and successfully utilized as efficient organocatalysts for a wide variety of asymmetric organic reactions. The utility of bifunctional catalysts can enhance catalytic activity and improve stereochemical control through their synchronous activation of both reaction partners. However, the NHCs possessing multiple activation sites are far less developed.This Account gives an overview of our research on the design, development, and applications of bifunctional NHCs in organocatalysis. We synthesized a series of l-pyroglutamic acid-derived bifunctional NHCs bearing a free hydroxyl group which can interact with carbonyl or imino groups via hydrogen-bonding. Further studies revealed that these bifunctional catalysts worked well for a variety of reactions. We have developed bifunctional NHC-catalyzed aza-benzoin reactions, [2 + 2], [2 + 3], and [2 + 4] cycloadditions of ketenes, [3 + 2] and [3 + 4] annulations of enals, and aza-MBH and Rauhut-Currier reactions of Michael acceptors. In addition to these reactions via nucleophilic Breslow intermediates, enolates, homoenolates, and zwitterionic azolium intermediates, the bifunctional NHC-catalyzed [3 + 3] annulation via 1,3-biselectrophilic α,β-unsaturated acyl azolium intermediates was also developed.In these reactions, bifunctional NHCs showed amazing effects compared to normal nonbifunctional NHCs. In some cases, the bifunctional NHCs facilitated reactions which did not work under normal NHC catalysis, possibly due to additional activation via H-bonding. More interestingly, the bifunctional NHCs could not only improve but also switch the enantioselectivity to get products with opposite stereochemistry through H-bond controlled stereochemical directing. Furthermore, the reaction mode could be totally changed from [3 + 2] to [3 + 4] annulation to give kinetically favored products when bifunctional NHCs were employed. In future, the applications of bifunctional NHCs in other challenging reactions, such as asymmetric reactions with carbon-carbon unsaturated bonds, and the reactions involving alkyl or heteroatom radicals will be the major focus in our group.
中文翻译:

衍生自1-焦谷氨酸的双功能N-杂环卡宾及其在对映选择性有机催化中的应用。
在自然界中,酶是构造对映体纯化学品的强大媒介,它总是激发合成化学家探索新的催化剂,以模仿酶机理进行不对称转化。维生素B1是双功能噻唑鎓N-杂环卡宾(NHC)的前体,是转酮醇酶的辅酶。在过去的二十年中,已经合成了一系列手性NHC,包括单环,双环,四环,甚至是桥连的NHC,并已成功地用作各种不对称有机反应的有效有机催化剂。双功能催化剂的实用性可以通过同时激活两个反应伙伴来增强催化活性并改善立体化学控制。但是,具有多个激活位点的NHC远远不够发达。该帐户概述了我们对双功能NHC在有机催化中的设计,开发和应用的研究。我们合成了一系列的l-焦谷氨酸衍生的双功能NHC,它们带有可以通过氢键与羰基或亚氨基相互作用的游离羟基。进一步的研究表明,这些双功能催化剂在各种反应中效果很好。我们开发了双功能NHC催化的氮杂安息香反应,烯酮的[2 + 2],[2 + 3]和[2 + 4]环加成,[3 + 2]和[3 + 4]环烯醛环化,以及迈克尔受体的aza-MBH和Rauhut-Currier反应。除了通过亲核Breslow中间体,烯醇化物,均烯酸酯和两性离子氮鎓中间体进行的这些反应之外,双官能NHC还通过1,3-双亲电子α催化[3 + 3]环化,还开发了β-不饱和酰基偶氮中间体,在这些反应中,双功能NHC与正常的非双功能NHC相比显示出惊人的效果。在某些情况下,双功能NHC促进了在正常NHC催化下不起作用的反应,这可能是由于通过H键的额外活化所致。更有趣的是,双官能NHCs不仅可以改善,而且可以通过H键控制的立体化学导向来转换对映选择性,从而获得具有相反立体化学的产物。此外,当使用双功能NHC时,反应模式可以完全从[3 + 2]更改为[3 + 4],得到动力学上有利的产物。将来,双功能NHC在其他挑战性反应中的应用,例如具有碳-碳不饱和键的不对称反应,
更新日期:2020-03-06
中文翻译:

衍生自1-焦谷氨酸的双功能N-杂环卡宾及其在对映选择性有机催化中的应用。
在自然界中,酶是构造对映体纯化学品的强大媒介,它总是激发合成化学家探索新的催化剂,以模仿酶机理进行不对称转化。维生素B1是双功能噻唑鎓N-杂环卡宾(NHC)的前体,是转酮醇酶的辅酶。在过去的二十年中,已经合成了一系列手性NHC,包括单环,双环,四环,甚至是桥连的NHC,并已成功地用作各种不对称有机反应的有效有机催化剂。双功能催化剂的实用性可以通过同时激活两个反应伙伴来增强催化活性并改善立体化学控制。但是,具有多个激活位点的NHC远远不够发达。该帐户概述了我们对双功能NHC在有机催化中的设计,开发和应用的研究。我们合成了一系列的l-焦谷氨酸衍生的双功能NHC,它们带有可以通过氢键与羰基或亚氨基相互作用的游离羟基。进一步的研究表明,这些双功能催化剂在各种反应中效果很好。我们开发了双功能NHC催化的氮杂安息香反应,烯酮的[2 + 2],[2 + 3]和[2 + 4]环加成,[3 + 2]和[3 + 4]环烯醛环化,以及迈克尔受体的aza-MBH和Rauhut-Currier反应。除了通过亲核Breslow中间体,烯醇化物,均烯酸酯和两性离子氮鎓中间体进行的这些反应之外,双官能NHC还通过1,3-双亲电子α催化[3 + 3]环化,还开发了β-不饱和酰基偶氮中间体,在这些反应中,双功能NHC与正常的非双功能NHC相比显示出惊人的效果。在某些情况下,双功能NHC促进了在正常NHC催化下不起作用的反应,这可能是由于通过H键的额外活化所致。更有趣的是,双官能NHCs不仅可以改善,而且可以通过H键控制的立体化学导向来转换对映选择性,从而获得具有相反立体化学的产物。此外,当使用双功能NHC时,反应模式可以完全从[3 + 2]更改为[3 + 4],得到动力学上有利的产物。将来,双功能NHC在其他挑战性反应中的应用,例如具有碳-碳不饱和键的不对称反应,











































 京公网安备 11010802027423号
京公网安备 11010802027423号