当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Analysis on the Role of Bicarbonate in Carbon Dioxide Electroreduction at Immobilized Cobalt Phthalocyanine
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-23 , DOI: 10.1021/acscatal.9b05272 Joy S. Zeng 1 , Nathan Corbin 1 , Kindle Williams 1 , Karthish Manthiram 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-23 , DOI: 10.1021/acscatal.9b05272 Joy S. Zeng 1 , Nathan Corbin 1 , Kindle Williams 1 , Karthish Manthiram 1
Affiliation
|
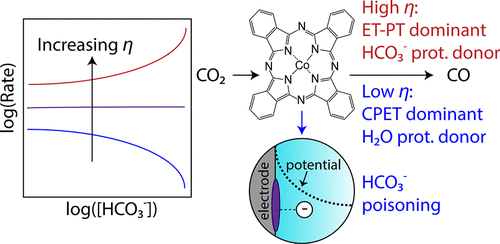
The mechanism for carbon dioxide reduction (CO2RR) to carbon monoxide (CO) at immobilized cobalt phthalocyanine (CoPc) in aqueous electrolytes has been widely debated. In this work, we investigated the mechanism of CO2RR to CO on CoPc via experimental reaction kinetics coupled with model fitting. Unexpectedly, reactant order dependences and Tafel slopes deviate from commonly expected values and change depending on the testing conditions. For example, (1) the effect of bicarbonate deviates from power law kinetics and transitions from inhibitory to promotional with increasingly reductive potential, and (2) the CO2 order dependence deviates from unity at more-reductive potentials. We propose a kinetic model, chosen from more than 15 candidate models, that is able to quantitatively fit all of the experimental data. The model invokes (1) catalyst poisoning via bicarbonate electrosorption, (2) mixed control between concerted proton–electron transfer (CPET) and sequential electron transfer-proton transfer (ET-PT), and (3) both water and bicarbonate as kinetically relevant proton donors. The proposed model also predicts that the relative importance of the above factors changes depending on the reaction conditions, highlighting the potential downfalls of broadly applying reaction mechanisms that were inferred from kinetic data collected in a narrow range of testing conditions. This study emphasizes the importance of cohesively using kinetic data collected over a wide range of operating conditions to test and formulate kinetic models of electrocatalytic reactions.
中文翻译:

碳酸氢盐在固定化酞菁钴二氧化碳电还原中的作用动力学分析
在水性电解质中固定化酞菁钴(CoPc)上二氧化碳还原(CO 2 RR)生成一氧化碳(CO)的机理已引起广泛争议。在这项工作中,我们通过实验动力学和模型拟合研究了CoPc上CO 2 RR转化为CO的机理。出乎意料的是,反应物顺序依赖性和Tafel斜率偏离了通常预期的值,并随测试条件而变化。例如,(1)碳酸氢盐的作用与幂律动力学背道而驰,从抑制作用向促进作用的转变具有越来越大的还原潜力,(2)CO 2在更多还原的电位上,顺序依赖性偏离了统一性。我们提出了一个动力学模型,该模型选自15种以上的候选模型,能够定量拟合所有实验数据。该模型调用(1)通过碳酸氢盐电吸附引起的催化剂中毒;(2)协调的质子-电子转移(CPET)和顺序电子转移-质子转移(ET-PT)之间的混合控制,以及(3)水和碳酸氢盐在动力学上相关质子供体。提出的模型还预测,上述因素的相对重要性会根据反应条件而变化,这突出表明了广泛应用的反应机理的潜在缺点,这些机理是根据在狭窄测试条件下收集的动力学数据推断出来的。
更新日期:2020-03-24
中文翻译:

碳酸氢盐在固定化酞菁钴二氧化碳电还原中的作用动力学分析
在水性电解质中固定化酞菁钴(CoPc)上二氧化碳还原(CO 2 RR)生成一氧化碳(CO)的机理已引起广泛争议。在这项工作中,我们通过实验动力学和模型拟合研究了CoPc上CO 2 RR转化为CO的机理。出乎意料的是,反应物顺序依赖性和Tafel斜率偏离了通常预期的值,并随测试条件而变化。例如,(1)碳酸氢盐的作用与幂律动力学背道而驰,从抑制作用向促进作用的转变具有越来越大的还原潜力,(2)CO 2在更多还原的电位上,顺序依赖性偏离了统一性。我们提出了一个动力学模型,该模型选自15种以上的候选模型,能够定量拟合所有实验数据。该模型调用(1)通过碳酸氢盐电吸附引起的催化剂中毒;(2)协调的质子-电子转移(CPET)和顺序电子转移-质子转移(ET-PT)之间的混合控制,以及(3)水和碳酸氢盐在动力学上相关质子供体。提出的模型还预测,上述因素的相对重要性会根据反应条件而变化,这突出表明了广泛应用的反应机理的潜在缺点,这些机理是根据在狭窄测试条件下收集的动力学数据推断出来的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号