当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Studies of Thioamide Effects on Serine Protease Activity Enable Two-Site Stabilization of Cancer Imaging Peptides.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-06 , DOI: 10.1021/acschembio.9b01036 Taylor M Barrett 1 , Xing S Chen 1 , Chunxiao Liu 1, 2 , Sam Giannakoulias 1 , Hoang Anh T Phan 1 , Jieliang Wang 1 , E Keith Keenan 1 , Richard J Karpowicz 1 , E James Petersson 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-06 , DOI: 10.1021/acschembio.9b01036 Taylor M Barrett 1 , Xing S Chen 1 , Chunxiao Liu 1, 2 , Sam Giannakoulias 1 , Hoang Anh T Phan 1 , Jieliang Wang 1 , E Keith Keenan 1 , Richard J Karpowicz 1 , E James Petersson 1
Affiliation
|
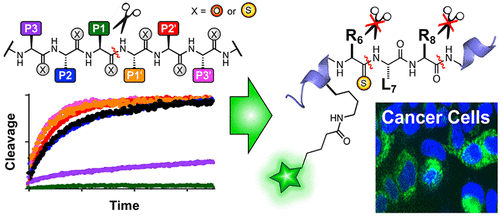
Thioamide substitutions in peptides can be used as fluorescence quenchers in protease sensors and as stabilizing modifications of hormone analogs. To guide these applications in the context of serine proteases, we here examine the cleavage of several model substrates, scanning a thioamide between the P3 and P3' positions, and identify perturbing positions for thioamide substitution. While all serine proteases tested were affected by P1 thioamidation, certain proteases were also significantly affected by other thioamide positions. We demonstrate how these findings can be applied by harnessing the combined P3/P1 effect of a single thioamide on kallikrein proteolysis to protect two key positions in a neuropeptide Y-based imaging probe, increasing its serum half-life to >24 h while maintaining potency for binding to Y1 receptor expressing cells. Such stabilized peptide probes could find application in imaging cell populations in animal models or even in clinical applications such as fluorescence-guided surgery.
中文翻译:

硫代酰胺对丝氨酸蛋白酶活性影响的研究能够实现癌症成像肽的两点稳定。
肽中的硫代酰胺取代可用作蛋白酶传感器中的荧光猝灭剂和激素类似物的稳定修饰。为了在丝氨酸蛋白酶的背景下指导这些应用,我们在这里检查了几种模型底物的切割,扫描了 P3 和 P3' 位置之间的硫代酰胺,并确定了硫代酰胺取代的扰动位置。虽然测试的所有丝氨酸蛋白酶都受到 P1 硫代酰胺化的影响,但某些蛋白酶也受到其他硫代酰胺位置的显着影响。我们展示了如何通过利用单一硫代酰胺对激肽释放酶蛋白水解的 P3/P1 组合效应来应用这些发现,以保护基于神经肽 Y 的成像探针中的两个关键位置,将其血清半衰期延长至 >24 小时,同时保持效力用于与 Y1 受体表达细胞结合。
更新日期:2020-03-06
中文翻译:

硫代酰胺对丝氨酸蛋白酶活性影响的研究能够实现癌症成像肽的两点稳定。
肽中的硫代酰胺取代可用作蛋白酶传感器中的荧光猝灭剂和激素类似物的稳定修饰。为了在丝氨酸蛋白酶的背景下指导这些应用,我们在这里检查了几种模型底物的切割,扫描了 P3 和 P3' 位置之间的硫代酰胺,并确定了硫代酰胺取代的扰动位置。虽然测试的所有丝氨酸蛋白酶都受到 P1 硫代酰胺化的影响,但某些蛋白酶也受到其他硫代酰胺位置的显着影响。我们展示了如何通过利用单一硫代酰胺对激肽释放酶蛋白水解的 P3/P1 组合效应来应用这些发现,以保护基于神经肽 Y 的成像探针中的两个关键位置,将其血清半衰期延长至 >24 小时,同时保持效力用于与 Y1 受体表达细胞结合。







































 京公网安备 11010802027423号
京公网安备 11010802027423号