当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bifunctional Hydrogen Bonding of Imidazole with Water Explored by Rotational Spectroscopy and DFT Calculations
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jpca.0c00544 Eva Gougoula 1 , Daniel J. Cole 1 , Nicholas R. Walker 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-03-23 , DOI: 10.1021/acs.jpca.0c00544 Eva Gougoula 1 , Daniel J. Cole 1 , Nicholas R. Walker 1
Affiliation
|
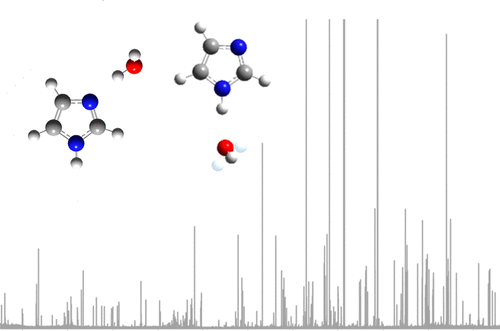
Laser vaporization of imidazole in the presence of an argon buffer gas has allowed the generation and isolation of two isomers of an imidazole monohydrate complex, denoted herein as imid···H2O and H2O···imid, within a gas sample undergoing supersonic expansion. Imidazole and water are respectively proton-accepting and proton-donating in imid···H2O, but these roles are reversed in the H2O···imid complex. Both isomers have been characterized by chirped-pulse Fourier transform microwave spectroscopy between 7.0 and 18.5 GHz. The ground-state rotational spectra of four isotopologues of imid···H2O and three isotopologues of H2O···imid have been measured. All spectra have been assigned and fitted to determine rotational (A0, B0, C0), centrifugal distortion (DJ, DJK), and nuclear quadrupole coupling constants (χaa(N1), [χbb(N1) – χcc(N1)], χaa(N3), and [χbb(N3) – χcc(N3)]). Structural parameters (r0 and rs) have been accurately determined from measured rotational constants for each isomer. The imid···H2O complex contains a nonlinear hydrogen bond (∠(O–Hb···N3) = 172.1(26)° in the experimentally determined, r0 geometry) between the pyridinic nitrogen of imidazole and a hydrogen atom of H2O. The DFT calculations find that the H2O···imid complex also contains a nonlinear hydrogen bond between the oxygen atom of water and the hydrogen attached to the pyrrolic nitrogen of imidazole (∠(O···H1–N1) = 174.7°). Two states observed in the spectrum of H2O···imid, assigned as 0– and 0+ states, confirm that large amplitude motions occur on the time scale of the molecular rotation. Density functional theory has been performed to characterize these large amplitude motions.
中文翻译:

旋转光谱法和DFT计算探讨咪唑与水的双功能氢键
在氩气缓冲气体存在下对咪唑进行激光汽化,可以在气体样品中生成和分离咪唑一水合物配合物的两个异构体,在本文中称为imid··H 2 O和H 2 O··imid经历超音速膨胀。咪唑和水在酰亚胺···H 2 O中分别为质子接受和质子给体,但在H 2 O···酰亚胺络合物中这些作用相反。两种异构体的特征都在于7.0至18.5 GHz之间的chi脉冲傅里叶变换微波光谱。的酰亚胺四个同位素基态转动谱···ħ 2 O和3个同位素异数体ħ 2O··imid已被测量。所有的光谱已经被分配并装配以确定旋转(甲0,乙0,Ç 0),离心失真(d Ĵ,d JK),和核四极耦合常数(χ AA(N1),[χ BB(N1) - χ立方厘米(N1)],χ AA(N3),并[χ BB(N3) - χ立方厘米(N3)])。结构参数(r 0和r s)已根据每种异构体的测量旋转常数精确确定。亚胺···H2 O配合物在咪唑的吡啶吡啶氮原子与H 2 O的氢原子之间包含一个非线性的氢键(根据实验确定的r(O–H b ··N3)= 172.1(26)°,r 0几何形状)。通过DFT计算发现,H 2 O···酰亚胺络合物在水的氧原子和与咪唑的吡咯氮原子相连的氢之间也包含非线性氢键(∠(O···H1-N1)= 174.7° )。在H 2 O···酰亚胺光谱中观察到的两个状态分别为0 –和0 +状态,请确认在分子旋转的时间尺度上发生了大幅度的运动。已经执行了密度泛函理论来表征这些大幅度运动。
更新日期:2020-03-24
中文翻译:

旋转光谱法和DFT计算探讨咪唑与水的双功能氢键
在氩气缓冲气体存在下对咪唑进行激光汽化,可以在气体样品中生成和分离咪唑一水合物配合物的两个异构体,在本文中称为imid··H 2 O和H 2 O··imid经历超音速膨胀。咪唑和水在酰亚胺···H 2 O中分别为质子接受和质子给体,但在H 2 O···酰亚胺络合物中这些作用相反。两种异构体的特征都在于7.0至18.5 GHz之间的chi脉冲傅里叶变换微波光谱。的酰亚胺四个同位素基态转动谱···ħ 2 O和3个同位素异数体ħ 2O··imid已被测量。所有的光谱已经被分配并装配以确定旋转(甲0,乙0,Ç 0),离心失真(d Ĵ,d JK),和核四极耦合常数(χ AA(N1),[χ BB(N1) - χ立方厘米(N1)],χ AA(N3),并[χ BB(N3) - χ立方厘米(N3)])。结构参数(r 0和r s)已根据每种异构体的测量旋转常数精确确定。亚胺···H2 O配合物在咪唑的吡啶吡啶氮原子与H 2 O的氢原子之间包含一个非线性的氢键(根据实验确定的r(O–H b ··N3)= 172.1(26)°,r 0几何形状)。通过DFT计算发现,H 2 O···酰亚胺络合物在水的氧原子和与咪唑的吡咯氮原子相连的氢之间也包含非线性氢键(∠(O···H1-N1)= 174.7° )。在H 2 O···酰亚胺光谱中观察到的两个状态分别为0 –和0 +状态,请确认在分子旋转的时间尺度上发生了大幅度的运动。已经执行了密度泛函理论来表征这些大幅度运动。











































 京公网安备 11010802027423号
京公网安备 11010802027423号