当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and Stereoselective Synthesis of 1,1-Diborylalkenes via Brønsted Base-Catalyzed Mixed Diboration of Alkynyl Esters and Amides with BpinBdan
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-03-18 , DOI: 10.1002/ejoc.202000128 Xiaocui Liu 1 , Wenbo Ming 1 , Xiaoling Luo 1, 2 , Alexandra Friedrich 1 , Jan Maier 1 , Udo Radius 1 , Webster L Santos 3 , Todd B Marder 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-03-18 , DOI: 10.1002/ejoc.202000128 Xiaocui Liu 1 , Wenbo Ming 1 , Xiaoling Luo 1, 2 , Alexandra Friedrich 1 , Jan Maier 1 , Udo Radius 1 , Webster L Santos 3 , Todd B Marder 1
Affiliation
|
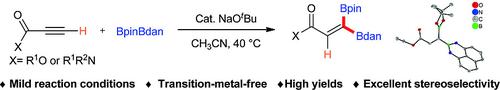
The NaOtBu‐catalyzed mixed 1,1‐diboration of terminal alkynes using the unsymmetrical diboron reagent BpinBdan (pin = pinacolato; dan = 1,8‐diaminonaphthalene) proceeds in a regio‐ and stereoselective fashion affording moderate to high yields of 1,1‐diborylalkenes bearing orthogonal boron protecting groups. It is applicable to gram‐scale synthesis without loss of yield or selectivity. The mixed 1,1‐diborylalkene products can be utilized in Suzuki–Miyaura cross‐coupling reactions which take place selectivly at the C–B site. DFT calculations suggest the NaOtBu‐catalyzed mixed 1,1‐diboration of alkynes occurs through deprotonation of the terminal alkyne, stepwise addition of BpinBdan to the terminal carbon followed by protonation with tBuOH. Experimentally observed selective formation of (Z)‐diborylalkenes is supported by our theoretical studies.
中文翻译:

通过 Brønsted 碱催化炔基酯和酰胺与 BpinBdan 的混合二硼化反应区域选择性和立体选择性合成 1,1-二硼基烯烃
使用不对称二硼试剂 BpinBdan(pin = pinacolato;dan = 1,8-二氨基萘),NaOtBu 催化末端炔烃的混合 1,1-二硼化反应以区域和立体选择性方式进行,提供中等到高产率的 1,1-带有正交硼保护基团的二硼基烯烃。它适用于克级合成,且不会损失产率或选择性。混合的 1,1-二硼基烯烃产物可用于选择性地在 C-B 位点发生的 Suzuki-Miyaura 交叉偶联反应。 DFT 计算表明,NaOtBu 催化的炔烃混合 1,1-二硼化是通过末端炔烃的去质子化、逐步将 BpinBdan 添加到末端碳、然后用 tBuOH 质子化来发生的。我们的理论研究支持了实验观察到的 (Z)-二硼基烯烃的选择性形成。
更新日期:2020-03-18
中文翻译:

通过 Brønsted 碱催化炔基酯和酰胺与 BpinBdan 的混合二硼化反应区域选择性和立体选择性合成 1,1-二硼基烯烃
使用不对称二硼试剂 BpinBdan(pin = pinacolato;dan = 1,8-二氨基萘),NaOtBu 催化末端炔烃的混合 1,1-二硼化反应以区域和立体选择性方式进行,提供中等到高产率的 1,1-带有正交硼保护基团的二硼基烯烃。它适用于克级合成,且不会损失产率或选择性。混合的 1,1-二硼基烯烃产物可用于选择性地在 C-B 位点发生的 Suzuki-Miyaura 交叉偶联反应。 DFT 计算表明,NaOtBu 催化的炔烃混合 1,1-二硼化是通过末端炔烃的去质子化、逐步将 BpinBdan 添加到末端碳、然后用 tBuOH 质子化来发生的。我们的理论研究支持了实验观察到的 (Z)-二硼基烯烃的选择性形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号