当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nidulaxanthone A, a xanthone dimer with a heptacyclic 6/6/6/6/6/6/6 ring system from Aspergillus sp.-F029
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-03-05 , DOI: 10.1039/d0qo00113a Fuqian Wang 1, 2, 3, 4 , Jie Jiang 1, 2, 3, 4 , Song Hu 1, 2, 3, 4 , Xincai Hao 5, 6, 7 , You-sheng Cai 4, 8, 9, 10, 11 , Ying Ye 12, 13, 14, 15, 16 , Haoran Ma 1, 2, 3, 4 , Weiguang Sun 12, 13, 14, 15, 16 , Lu Cheng 1, 2, 3, 4 , Chuanqi Huang 1, 2, 3, 4 , Hucheng Zhu 12, 13, 14, 15, 16 , Hong Zhang 1, 2, 3, 4 , Geng Zhang 1, 2, 3, 4 , Yonghui Zhang 12, 13, 14, 15, 16
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-03-05 , DOI: 10.1039/d0qo00113a Fuqian Wang 1, 2, 3, 4 , Jie Jiang 1, 2, 3, 4 , Song Hu 1, 2, 3, 4 , Xincai Hao 5, 6, 7 , You-sheng Cai 4, 8, 9, 10, 11 , Ying Ye 12, 13, 14, 15, 16 , Haoran Ma 1, 2, 3, 4 , Weiguang Sun 12, 13, 14, 15, 16 , Lu Cheng 1, 2, 3, 4 , Chuanqi Huang 1, 2, 3, 4 , Hucheng Zhu 12, 13, 14, 15, 16 , Hong Zhang 1, 2, 3, 4 , Geng Zhang 1, 2, 3, 4 , Yonghui Zhang 12, 13, 14, 15, 16
Affiliation
|
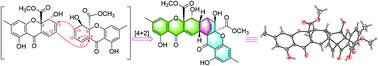
Nidulaxanthone A (1), a xanthone dimer bearing an unprecedented heptacyclic 6/6/6/6/6/6/6 system, together with a new monomeric nidulalin D (2) and four known analogues (3, 4, 5 and 6), were isolated from Aspergillus sp. F029. Biosynthetically, 1 was likely generated via [4 + 2] cycloaddition of precursor 3. Compounds 1, 2, and 3 exhibited cytotoxicity with IC50 values ranging from 3.2 to 21.9 μM, and antibacterial activity with MIC50 values of 13.2 and 19.6 μg ml−1 for 2 and 3, respectively.
中文翻译:

Nidulaxanthone A,一种具有七环6/6/6/6/6/6/6环系统的黄酮二聚体,来自Aspergillus sp.-F029
Nidulaxanthone A(1),呫吨酮二聚体轴承前所未有的七环6/6/6/6/6/6/6系统,一起用新的单体nidulalin d(2)和四个已知类似物(3,4,5和6),分离自曲霉菌。F029。生物合成,1很可能产生通过[4 + 2]的前体的环加成3。化合物1,2,和3与IC展出的细胞毒性50值范围从3.2至21.9微米,并且抗菌活性与MIC 50分别为2和3的13.2和19.6μgml -1值。
更新日期:2020-04-24
中文翻译:

Nidulaxanthone A,一种具有七环6/6/6/6/6/6/6环系统的黄酮二聚体,来自Aspergillus sp.-F029
Nidulaxanthone A(1),呫吨酮二聚体轴承前所未有的七环6/6/6/6/6/6/6系统,一起用新的单体nidulalin d(2)和四个已知类似物(3,4,5和6),分离自曲霉菌。F029。生物合成,1很可能产生通过[4 + 2]的前体的环加成3。化合物1,2,和3与IC展出的细胞毒性50值范围从3.2至21.9微米,并且抗菌活性与MIC 50分别为2和3的13.2和19.6μgml -1值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号