当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Room temperature nickel-catalyzed cross-coupling of aryl-boronic acids with thiophenols: synthesis of diarylsulfides
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/05 , DOI: 10.1039/d0ob00244e Amit Bhowmik 1, 2, 3, 4 , Mahesh Yadav 1, 2, 3, 4 , Rodney A. Fernandes 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020/03/05 , DOI: 10.1039/d0ob00244e Amit Bhowmik 1, 2, 3, 4 , Mahesh Yadav 1, 2, 3, 4 , Rodney A. Fernandes 1, 2, 3, 4
Affiliation
|
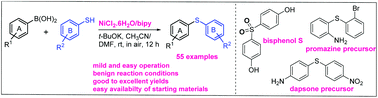
A NiCl2/2,2′-bipyridine-catalyzed cross-coupling of thiophenols with arylboronic acids has been developed for the synthesis of symmetric and unsymmetric diarylsulfides at room temperature and in air. This methodology is reliable and offers a mild and easy to operate process for the synthesis of arylthioethers, which are essential compounds with applications in the pharmaceutical and agricultural industries. This method avoids the use of expensive transition metals, such as Pd, Ir or Rh, sophisticated ligands and elevated temperatures. It also has a wide substrate scope (55 examples) and provides products in good to excellent yields (72–93%).
中文翻译:

室温镍催化的芳基硼酸与硫酚的交叉偶联:二芳基硫醚的合成
已经开发了NiCl 2 / 2,2'-联吡啶催化的硫代酚与芳基硼酸的交叉偶联,用于在室温和空气中合成对称和不对称的二芳基硫化物。该方法学可靠,为芳基硫醚的合成提供了温和且易于操作的方法,芳基硫醚是在制药和农业行业中应用的必不可少的化合物。该方法避免了使用昂贵的过渡金属,例如Pd,Ir或Rh,复杂的配体和高温。它还具有广泛的底物范围(55个示例),并提供了良率至良率(72-93%)的产品。
更新日期:2020-04-01
中文翻译:

室温镍催化的芳基硼酸与硫酚的交叉偶联:二芳基硫醚的合成
已经开发了NiCl 2 / 2,2'-联吡啶催化的硫代酚与芳基硼酸的交叉偶联,用于在室温和空气中合成对称和不对称的二芳基硫化物。该方法学可靠,为芳基硫醚的合成提供了温和且易于操作的方法,芳基硫醚是在制药和农业行业中应用的必不可少的化合物。该方法避免了使用昂贵的过渡金属,例如Pd,Ir或Rh,复杂的配体和高温。它还具有广泛的底物范围(55个示例),并提供了良率至良率(72-93%)的产品。









































 京公网安备 11010802027423号
京公网安备 11010802027423号